Home > Healthcare > Pharmaceuticals > Disease Specific Drugs > Hemophilia Treatment Market
Hemophilia Treatment Market Analysis
- Report ID: GMI2772
- Published Date: Feb 2022
- Report Format: PDF
Hemophilia Treatment Market Analysis
Based on disease, the hemophilia treatment market is segmented as hemophilia A and hemophilia B. Hemophilia A disease segment dominated the market in 2021 accounting for around 82% of the total revenue share. This high revenue share of the segment is owing to an increased incidence of hypertension among hemophilia A patients, favourable government initiatives for disease control, and surging disease awareness among the population among others.
As per the Centers for Disease Control and Prevention (CDC) recent data, around 400 babies are born with hemophilia A each year in the U.S. This considerable disease incidence is expected to drive the demand for hemophilia A treatment, thereby propelling the segmental revenue positively. The National Hemophilia Foundation (NHF) is actively working to upsurge the awareness pertaining to symptoms, genetics, diagnosis, and treatment of hemophilia A.
Based on product, the hemophilia treatment market is segmented as recombinant factor concentrates, plasma-derived factor concentrates, extended half-life products, and others. The recombinant factor concentrates segment is expected to expand at a CAGR of around 5% from 2022-2028. This significant share of the segment is due to the several advantages delivered by recombinant factor concentrates in hemophilia treatment.
The recombinant factor VIII concentrate provides a technological solution to prolonging the half-life of and decreasing the risk of formation of alloantibodies (inhibitors) against FVIII in treated patients with hemophilia A (HA). All rFVIII products have a better-quality safety profile, as their development process involves virus inactivation procedures similar to those used in plasma-derived factor concentrates.
For patients with severe hemophilia, prophylaxis with FVIII concentrates is the preferred hemophilia treatment to lessen joint bleeding and other types of hemorrhage, thereby enhancing the health-related quality of life of patients. Thus, the above-mentioned factors will stimulate industry landscape.
Based on patient, the market is segmented as pediatric and adult. The adult patient segment exceeded USD 6,898 million in 2021. The prevalence of hemophilia is significantly increasing among the adult population. As per the recent study data, during the period 2012-2018, around 33,000 males were suffering from hemophilia in the U.S. As the age of patients increases, the requirement for quality care to treat this disorder also increases. Thus, several nations are focusing to improve access to hemophilia diagnosis and treatment.
Further, improvement of the global comprehensive care offered by specialized centers is stimulating the adoption of hemophilia treatment, thereby driving the market revenue. Recent advancements in treatment options have significantly improved the life expectancy of adult patients. Thus, numerous advantages coupled with treatment advancements will surge the adoption of the product among adult patients, thereby amplifying the hemophilia treatment industry share.
Based on treatment, the hemophilia treatment market is segmented as prophylaxis and on demand. The prophylaxis segment exceeded USD 7,998 million in 2021. Prophylaxis treatment is considered as the standard of care for treating factor VIII deficiency in pediatric and adult patients. It involves the administration of clotting factor concentrate to prevent bleeding.
This prophylaxis treatment aids in reducing hemophilic arthropathy & disability, decreasing the requirement for orthopedic surgery, thereby delivering improved quality of life. The majority healthcare providers opt for this treatment option attributed to its efficiency and better clinical outcomes. The developments of prophylactic regimens of factor concentrate infusions allowed their administration at home, thereby positively propelling the treatment acceptance. Hence, the above-mentioned factors are expected to facilitate market expansion over the estimation timeframe.
Based on therapy, the market is segmented as factor replacement therapy and non-factor replacement therapy. The non-factor replacement therapy accounted for more than USD 1,648 million in 2021 and is projected to witness over 21.3% CAGR through 2028. This high growth rate is due to the advantages non-factor replacement therapy offers that include reducing treatment burden, increasing the ability to deliver prophylaxis for patients, etc. Limited real-world data and authenticated practical guidance on these recently licensed treatments resulted in increasing their application in hemophilia therapy.
As per the National Center for Biotechnology Information (NCBI), Emicizumab is currently the only licensed nonfactor therapy that helps with venous access issues, frequent bleeds, etc. This therapy is also suggested to prevent bleeds in patients with inhibitors undergoing surgery. Owing to recent advancements, these novel agents have promising hemostatic properties and supports marked reductions of bleeding episodes in hemophilia patients with or without inhibitors.
Thus, the major players in the hemophilia treatment market focuses on developing such advanced therapies with potential advantages comprising marked suppression of the onset of arthropathy. Hence, the increased acceptance of non-factor replacement therapy is predicted to boost the industry expansion.
Based on drug class, the hemophilia treatment market is segmented as vasopressin and coagulation factors. The vasopressin segment accounted for more than USD 573 million in 2021 and is projected to witness 6% CAGR rate till 2028. This considerable segmental share is owing to the improved survival chances in critical patients with the use of vasopressin. As per the National Hemophilia Foundation, this drug can be used for nose & mouth bleeds, joint & muscle bleeds, and before & after surgery among others. It can be administered through both intravenous and nasal routes, thereby reducing the systemic adverse effects of the drugs.
DDAVP (desmopressin acetate) is the synthetic form of vasopressin, a natural antidiuretic hormone that aids stop bleeding due to its clinical efficacy & safety and the availability of concentrated formulation for the intravenous and nasal route of drug administration. As a result, several advantages offered by the use of vasopressin are set to boost the segmental growth.
Based on route of administration, the market is segmented as injectable and nasal spray. The injectables segment is expected to witness over4.9% CAGR between 2022 and 2028. This significant segment growth is due to the fast-acting and efficient mode of drug administration delivered by injectables. This route of administration is recommended for patients that witness challenging circumstances associated with oral intake of medicines.
Intravenous injection route in hemophilia treatment has related to enhancing clinical outcomes, surging the life expectancy, functional status, and quality of life among others. Thus, the majority of the medications and factor concentrate products are intravenously given to patients for improved and quick outcomes.

Based on end-use, the hemophilia treatment market is segmented as hospitals, clinics, hemophilia treatment centers, and others. The hospitals segment accounted for USD 3,382 million in 2021 attributed to the accessibility to board-certified skilled hematologists, the importance of delivering quality care plans, and their improved efficiency among others. These healthcare facilities provide affordable treatment procedures that help both patients and insurers equally.
Easy availability of novel therapies surges the preference for bleeding disorder treatment in hospitals. Also, comprehensive care plans and continuous monitoring offered in hospitals are anticipated to increase the patient preference for these healthcare facilities. Thus, the growing hospital admissions with such inherited bleeding ailments and demand for advanced treatments are projected to fuel the adoption of the product in these facilities.
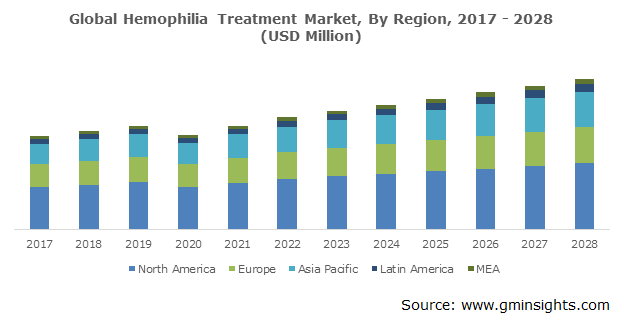
U.S. hemophilia treatment market is poised to surpass USD 6,297 million by 2028. This high growth in the country is owing to several factors including increased government spending on healthcare and R&D activities along with the presence of established manufacturers. Surging awareness related to technologically advanced products and rising target patient pool is also contributing to the business revenue. As per the World Federation of Hemophilia (WFH), in 2018, around 17,757 individuals were diagnosed with hemophilia in the U.S. The organization comprising Hemophilia Federation of America (HFA), commonly delivers continuous treatment for patients by presenting different co-pay initiatives.
Further, in 2021, BioMarin Pharmaceutical Inc. announced a significant Phase 3 gene therapy trial results in adults with severe hemophilia A in the U.S. Such ongoing R&D activities are expected to fuel the market demand over the forecast period. As a result, the above-mentioned factors are anticipated to augment the product adoption in the country.

