Home > Healthcare > Medical Services > Lab Services > Medical Device Testing Services Market
Medical Device Testing Services Market Size
- Report ID: GMI6237
- Published Date: Jul 2023
- Report Format: PDF
Medical Device Testing Services Market Size
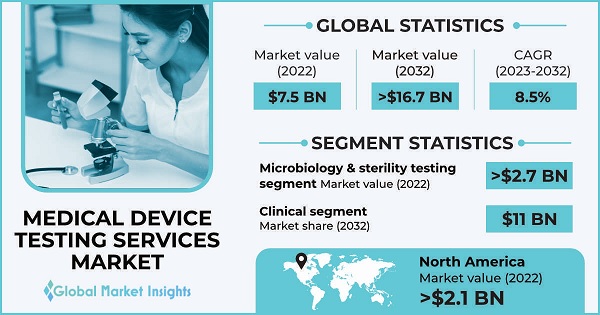
Medical Device Testing Services Market size was valued at around USD 7.5 billion in 2022 and it is anticipated to reach over 16.7 billion by 2032 at a CAGR of 8.5%. Rising complexity of medical devices, advancements in testing technologies, stringent regulatory requirements and focus on patient safety among others are key trends shaping the market growth.
As medical devices become more advanced and complex, their testing requirements also become more intricate. Manufacturers often lack the resources and expertise to perform these specialized tests in-house, leading them to rely on third-party testing services. Additionally, healthcare professionals, including doctors and surgeons, are becoming more conscious of the importance of using approved and tested medical devices. As a result, they are more likely to recommend and use devices that have undergone thorough testing and certification.
| Report Attribute | Details |
|---|---|
| Base Year: | 2022 |
| Medical Device Testing Services Market Size in 2022: | 7.5 Billion USD |
| Forecast Period: | 2023 to 2032 |
| Forecast Period 2023 to 2032 CAGR: | 8.5% |
| 2032 Value Projection: | 16.7 Billion USD |
| Historical Data for: | 2018 to 2022 |
| No. of Pages: | 130 |
| Tables, Charts & Figures: | 190 |
| Segments covered: | Services, Phase and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
Patient safety is of utmost importance in the healthcare industry. Thorough testing of medical devices helps identify potential risks and ensures that only safe and effective products reach the market, benefiting patients and healthcare providers. As the medical industry continues to evolve, the need for reliable and comprehensive testing will remain a critical factor for ensuring patient safety and market success for manufacturers.
Medical device testing services refer to the comprehensive range of tests and evaluations conducted on medical devices to ensure their safety, efficacy, and compliance with regulatory standards before they are approved for use in the market. These services are crucial in the medical industry as they help identify potential risks associated with the devices and confirm their performance and reliability.
However, while stringent regulations can drive demand for testing services, they can also create obstacles for some manufacturers. Meeting complex regulatory requirements can be time-consuming and costly, particularly for companies operating in multiple countries with varying regulatory frameworks. Comprehensive testing of medical devices can be expensive, especially when using advanced technologies and specialized equipment. This cost burden may deter some manufacturers, particularly smaller ones, from seeking professional testing services and could lead to delays in bringing products to market.
Further, some medical device manufacturers, particularly smaller or newer companies, may not fully understand the importance of comprehensive testing or the regulatory requirements. This lack of awareness can hinder the growth of the market.