Home > Healthcare > Biotechnology > Biopharma > Immunoglobulin Market
Immunoglobulin Market Analysis
- Report ID: GMI5752
- Published Date: Sep 2023
- Report Format: PDF
Immunoglobulin Market Analysis
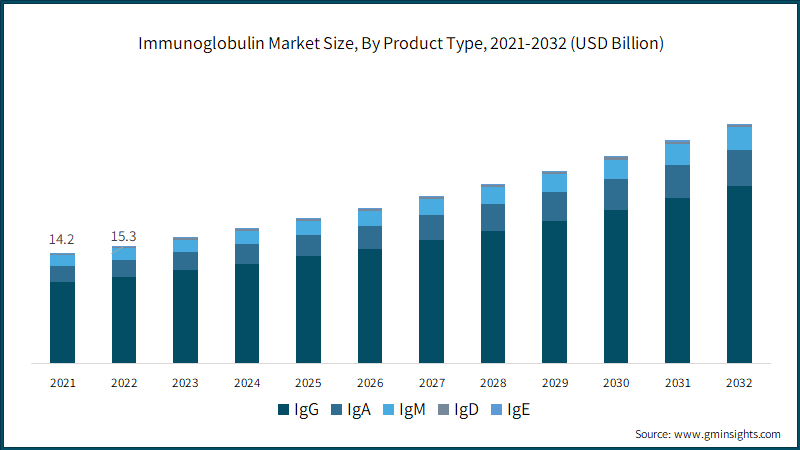
Based on product type, the immunoglobulin market is segmented as IgG, IgA, IgM, IgD, and IgE. The IgG segment accounted around 74% of the business share in 2022 and is projected to witness robust growth over the analysis timeframe. During the projected period, market growth is expected to be driven by the increasing acceptance of immunoglobulins, a growing frequency of product launches, and swift approvals from governmental regulatory bodies at various levels.
For instance, in January 2022, Argenx SE, a global immunology company, announced the approval of VYVGART by Japan's Ministry of Health, Labour and Welfare (MHLW). VYVGART, administered through intravenous infusion (efgartigimod alfa), is indicated for the treatment of adult patients with generalized myasthenia gravis (gMG) who have not responded adequately to steroids or non-steroidal immunosuppressive therapies (ISTs). Thus, increasing number of product approvals is expected to nurture market expansion. Another contributing factor to the global market's growth is the progression of government initiatives that endorse the intravenous delivery of medications.

Based on route of administration, the immunoglobulin market is segmented as intravenous (IVIG), and subcutaneous (SCIg). The intravenous (IVIG) held a dominant business share of around 71.2% in 2022 and is expected to grow at a significant pace during the analysis period. The rapid absorption rate provided by intravenous mode of delivery and the high immunoglobulin bioavailability are the factors supporting the expansion of the intravenous mode of delivery market. Furthermore, numerous immunoglobulins are receiving more financing, which is fostering the market's expansion.
For instance, the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is sponsoring and funding the Phase 3 trial, called Inpatient Treatment with Anti-Coronavirus Immunoglobulin, or ITAC. The antibody solution being tested in the ITAC trial is anti-coronavirus hyperimmune intravenous immunoglobulin, or hIVIG. Such factor will accelerate the market growth. Moreover, increasing application of plasma derived therapy for the treatment of rare diseases will further escalate the market expansion.
Based on application, the immunoglobulin market is segmented as chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MMN), primary immunodeficiency disease (PID), secondary immunodeficiency disease (SID), Guillain-Barre syndrome, immune thrombocytopenic purpura (ITP) and others. The chronic inflammatory demyelinating polyneuropathy (CIDP) segment is expected to grow at a steady pace of 8% between 2023 to 2032. The market is set to experience accelerated growth due to the increasing incidence of chronic inflammatory demyelinating polyneuropathy (CIDP). For instance, CIDP's overall prevalence is estimated to range from 4.8 to 8.9 cases per 100,000 individuals, with a lower estimated prevalence of 0.5 cases per 100,000 persons among children. CIDP treatment typically involves the use of immunosuppressants, steroids, and plasmapheresis.
However, intravenous immunoglobulin (IVIG) emerges as an effective long-term alternative to these methods, thereby serving as a catalyst for market expansion. Furthermore, the associated advantages, such as safety, minimally invasive procedures, and user-friendly treatment options, are expected to contribute significantly to the growth of the industry.
Based on end-use, the immunoglobulin market is segmented as hospitals, clinics, and homecare. The hospitals segment held a dominant market share in 2022 and is expected to grow at a significant pace of 8.1% between 2023 to 2032. The segment's predominance can be chiefly attributed to its advanced medical infrastructure and continuous medical supervision provided by healthcare professionals around the clock. Additionally, the expanding healthcare facilities catering to the health needs of both developed and emerging nations have led to a rise in the number of patients receiving hospital care.
For instance, data from the American Hospital Association (AHA) in 2022 reveals that the U.S. had a total of 6,129 hospitals with a cumulative 3.4 million hospital admissions. As a result, the increasing count of patients seeking hospitalization services is poised to bolster growth within this segment.

U.S. immunoglobulin market exceeded USD 7.6 billion in 2022. The business share in this region can be attributed to several factors, including the increasing healthcare spending, growing awareness about products used in the treatment of immunodeficiency disorders, and the rising preference among physicians for immunodeficiency therapies. Moreover, the market is expected to benefit from the expanding regulatory approvals for enhancing the manufacturing processes of immunoglobulins.
For example, in April 2021, ADMA Biologics, Inc., a biopharmaceutical company specializing in the production, promotion, and development of specialized plasma-derived biologics, announced that it had received approval from the U.S. FDA for an expanded manufacturing process. This approval enables the fractionation and purification of a 4,400-liter plasma pool for the production of intravenous immune globulin ("IVIG"). Such regulatory clearance is projected to stimulate market demand.