Home > Healthcare > Biotechnology > Bioservices > Clinical Trial Management System Market
Clinical Trial Management System Market Size
- Report ID: GMI1155
- Published Date: Jul 2022
- Report Format: PDF
Clinical Trial Management System Market Size
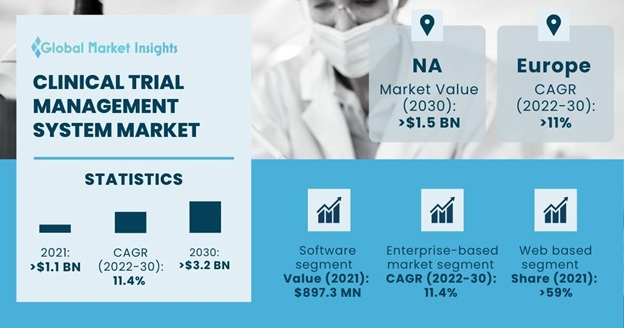
Clinical Trial Management System Market size accounted for about USD 1.2 billion in 2021 and is estimated to grow with a 11.4% market CAGR between 2022 to 2030, owing to the rising focus of the healthcare sector in research and development activities worldwide.
Many large-scale pharmaceutical and biotech industry participants are noted to be engaged in constant research in order to develop novel products as well as to sustain in the market. For instance, according to the UNESCO Institute for Statistics (UIS), in 2021, the global R&D investment surpassed USD 1.7 trillion. Around ten countries accounted for nearly 80% of all R&D spending.
| Report Attribute | Details |
|---|---|
| Base Year: | 2021 |
| Clinical Trial Management System Market Size in 2021: | USD 1,169.9 Million |
| Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 11.4% |
| 2030 Value Projection: | USD 3,202.7 Million |
| Historical Data for: | 2017 to 2021 |
| No. of Pages: | 150 |
| Tables, Charts & Figures: | 225 |
| Segments covered: | Component, Product, Delivery Mode, End-use and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
Several countries have committed to further enhance the public and corporate R&D spending, as well as the number of researchers, by 2030 as part of the Sustainable Development Goals (SDGs). Furthermore, one of the primary value drivers for research-based pharmaceutical businesses is innovative medications addressing the unmet medical needs. Owing to enormous growth in R&D activities, having essential tools to plan, manage and track clinical studies effectively is need of the hour. Hence, eClinical solutions, such as clinical trial management system (CTMS), are gaining market prominence.
The emergence of the COVID-19 pandemic significantly impacted the overall healthcare sector, disrupting the activities of virtually every organization operating within. To conduct clinical trials smoothly in healthcare and life science industry, where physical access to patients was not possible, using new ways for patient monitoring and caring had become necessary. Due to which the previously present data collection methods, CTMS which were not utilized before, were quickly adopted in clinical trials during COVID outbreak.
Although, these means were available before, the pandemic acted as a catalyst in their increased market usage. With the introduction of CTMS, new approaches and technology in clinical trials specifically the one’s related to remote data collection from patients increased the data variety and volume. As a result, majority positive impacts of clinical trial approaches newly implemented during the pandemic were due to the data. The respondents using CTMS and other new methods of data collection in clinical trials found their data to be well timed, accurate, frequent, and more robust. However, many clinical trials were suspended or postponed due to imposed lockdowns and other restrains due to government rules