Summary
Table of Content

Rapid Diagnostics Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Rapid Diagnostics Market Size
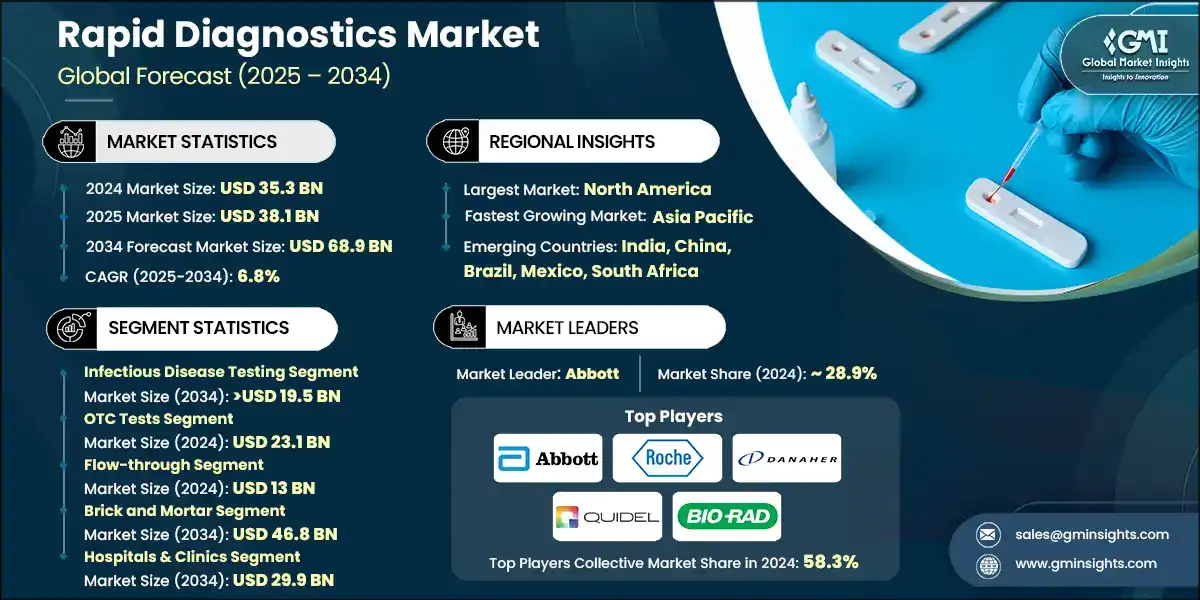
The global rapid diagnostics market was valued at USD 35.3 billion in 2024. The market is expected to reach from USD 38.1 billion in 2025 to USD 68.9 billion in 2034, growing at a CAGR of 6.8% during the forecast period, according to the latest report published by Global Market Insights Inc. The high market growth is attributed to the rising prevalence of infectious diseases, growing demand for point-of-care testing (POCT), technological advancements in diagnostic platforms, and increasing investment and funding in healthcare infrastructure, among other contributing factors.
To get key market trends
Rapid diagnostics refer to medical tests that can detect diseases, infections, or any other health conditions in a very short time (mostly within minutes to a few hours). They are extensively used in point-of-care settings such as clinics, pharmacies, and homes for timely decision-making. Such tests use methods like lateral flow assays, molecular diagnostics, and immunoassays to provide quick, dependable, and usable results.
The major companies that dominate the market of rapid diagnostics include Abbott, Roche, Danaher, Quidel, and BIO-RAD. These firms keep their competitive advantage by the continuous innovation of their products, global presence in different markets, and considerable investment in research and development.
The market has increased from USD 28.1 billion in 2021 and reached USD 32.7 billion in 2023, with the historic growth rate of 7.6%. The market growth was driven by the rising prevalence of infectious diseases, increased demand for point-of-care testing, and the widespread adoption of home-based self-diagnostic kits.
Moreover, the rising global prevalence of infectious diseases including HIV, influenza, malaria, and COVID-19 has increased the demand for rapid diagnostics. For instance, according to WHO, in 2023, there were 39 million people living with HIV globally, while malaria caused 619,000 deaths in 2021. These diagnostic tests enable early detection and prompt isolation of infected individuals, helping prevent disease transmission.
Government agencies and non-governmental organizations continue to invest in diagnostic programs, with the U.S. government allocating USD 47.5 billion in 2023 for COVID-19 testing initiatives. The Global Fund committed USD 14.7 billion between 2021-2024 for HIV, TB, and malaria diagnostic and treatment programs, which supports wider adoption of these tests.
Furthermore, innovations in molecular diagnostics, biosensors, and microfluidics have enhanced the accuracy and efficiency of rapid diagnostic tests. Integration with digital platforms and AI-powered analysis further supports faster results. Such advancements improve reliability and broaden applications beyond infectious disease detection.
Rapid Diagnostics Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 35.3 Billion |
| Market Size in 2025 | USD 38.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 6.8% |
| Market Size in 2034 | USD 68.9 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of infectious disease | The surge in infectious diseases like COVID-19, influenza, and malaria continues to fuel strong demand for rapid diagnostics to enable timely detection and containment. |
| Technological advancements | Innovations in molecular diagnostics, lateral flow assays, and digital integration are enhancing test speed, sensitivity, and usability, expanding adoption across care settings. |
| Growing popularity of rapid diagnostic tests | Their convenience, affordability, and faster turnaround compared to conventional lab testing are boosting acceptance among healthcare providers and consumers. |
| Rising awareness regarding early diagnosis among people | Public health campaigns and preventive healthcare trends are encouraging individuals to seek early testing, driving consistent market uptake. |
| Increasing government initiatives for the diagnosis of infectious diseases | Subsidies, procurement programs, and public-private partnerships are increasing accessibility and deployment in both developed and emerging regions. |
| Pitfalls & Challenges | Impact |
| Low accuracy of results | Variability in sensitivity and specificity raises concerns among clinicians, limiting adoption in critical diagnostic decisions. |
| Availability of substitute products | Centralized laboratory tests and advanced imaging solutions remain preferred in some settings, posing competition to rapid diagnostics. |
| Stringent regulatory framework | • Complex approval pathways and compliance requirements slow down product launches and market expansion, especially for innovative test formats. |
| Opportunities: | Impact |
| Expansion into emerging markets and decentralized care sites | Growing healthcare infrastructure and rising affordability in emerging economies will open large-scale adoption, especially in rural and resource-limited settings. |
| Integration with telemedicine and digital reporting | Linking rapid tests with virtual care platforms and cloud-based result sharing will enhance accessibility, improve patient monitoring, and create new revenue streams. |
| Market Leaders (2024) | |
| Market Leaders |
~ 28.9% market share |
| Top Players |
Collective market share in 2024 is Collective Market Share 58.3% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | India, China, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Rapid Diagnostics Market Trends
The market is growing considerably with the shift toward molecular and genetic rapid tests, growth of at-home and self-testing kits, integration with digital health and mobile apps, and expansion into non-communicable diseases (NCDs), among other factors collectively driving industry growth.
- Rapid diagnostics are increasingly connected with smartphones and digital platforms for data storage and result interpretation. This integration supports remote monitoring and telemedicine services. It also helps healthcare providers track patient outcomes in real time, enabling better disease management.
- Moreover, the adoption of molecular diagnostics is increasing due to the technology’s higher sensitivity and specificity. The National Institutes of Health report (NIH) states that the volume of tests for molecular diagnostics has surged by 32% between 2021 and 2023, with a total number of over 850 million tests globally in 2023. The advent of PCR and other isothermal amplification methods has brought about the speed and portability of molecular tests.
- In 2023, the FDA approved 47 new molecular diagnostic tests, that is 15% more than in 2022. As a result of these developments, molecular diagnostics have moved beyond infectious diseases testing to be used in oncology and genetic screening applications, with oncology molecular diagnostics segment being on a trajectory of 8.2% annual growth since 2021.
- Additionally, healthcare systems are prioritizing rapid diagnostics in rural, low-resource, and emergency environments. Portable, battery-operated devices and low-cost test kits are enabling wider access. This trend aligns with global health goals to improve diagnostics equity and strengthen primary healthcare, thereby sustaining market growth.
Rapid Diagnostics Market Analysis
Learn more about the key segments shaping this market
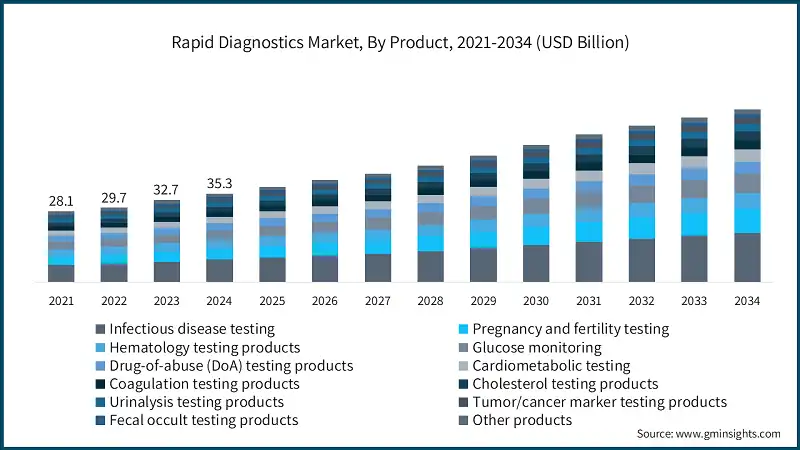
Based on the product, the rapid diagnostics market is segmented into infectious disease testing, pregnancy and fertility testing, hematology testing products, glucose monitoring, drug-of-abuse (DoA) testing products, cardiometabolic testing, coagulation testing products, cholesterol testing products, urinalysis testing products, tumor/cancer marker testing products, fecal occult testing products, and other products.
The infectious disease testing segment has asserted its dominance in the market by securing a significant market share of 25.2% in 2024 owing to the rising global burden of infectious diseases, increasing demand for rapid detection, and widespread adoption of point-of-care testing solutions. The segment is expected to exceed USD 19.5 billion by 2034, growing at a CAGR of 8.1% during the forecast period.
On the other hand, the pregnancy and fertility testing segment is expected to grow with a CAGR of 8.8%. The growth of this segment is driven by increasing consumer preference for home-based self-testing kits, rising awareness about family planning, and growing accessibility through retail and online channels.
- The infectious disease testing segment continues to dominate the market. The increasing prevalence of infectious diseases such as HIV, tuberculosis, influenza, malaria, and hepatitis. Rapid diagnostics allow early identification and are the cause of timely treatment, thus lowering the death rate. The growing number of cases of these diseases is the main source of the market for fast and reliable testing solutions.
- Additionally, infectious diseases often spread rapidly, making timely diagnosis critical. Rapid tests are increasingly deployed in clinics, airports, and community health centers, enabling immediate decision-making. This decentralization is especially vital in resource-limited and remote settings where laboratory facilities are scarce.
- The pregnancy and fertility testing segment held a revenue of USD 4.2 billion in 2024, with projections indicating a steady expansion at 8.8% CAGR from 2025 to 2034. The segment is driven by the increasing emphasis on reproductive health and the role of early detection in family planning. The surge in consumer adoption is a result of the rising availability of easy-to-use and affordable home testing kits. Furthermore, the growing sales via pharmacies and e-commerce platforms are attracting more customers.
- The hematology testing products segment held a revenue of USD 3.7 billion in 2024, with projections indicating a steady expansion at 4.9% CAGR from 2025 to 2034. The segmental growth is driven by the rising prevalence of blood disorders such as anemia and leukemia, creating strong demand for quick screening solutions. Advancements in portable hematology analyzers and the need for rapid blood parameter monitoring in emergency and critical care settings further support market growth.
- The glucose monitoring segment accounted for significant revenue in 2024, with projections indicating a steady expansion at 7.6% CAGR from 2025 to 2034. The growth of the segment is driven by the increasing global burden of diabetes and the growing emphasis on continuous, real-time glucose tracking for better disease management. Expanding adoption of wearable and connected glucose monitoring devices is further accelerating demand across home care and clinical settings.
- The drug-of-abuse (DoA) testing products segment held a revenue of USD 3 billion in 2024, with projections indicating a steady expansion at 4.5% CAGR from 2025 to 2034. The segment is driven by rising concerns over substance abuse in workplaces, schools, and law enforcement. The need for instant, on-site results and the growing adoption of non-invasive testing methods are further propelling market demand.
Based on purchase, the rapid diagnostics market is segmented into OTC tests and prescription-based tests. The OTC tests segment dominated the market in 2024, accounting for USD 23.1 billion and is anticipated to grow at a CAGR of 7.1% during the forecast period.
- Health-conscious consumers are proactively managing their healthcare, which is the main reason for the elevated demand of self-testing solutions. The promotional activities and digital health information make the users more confident in using OTC diagnostic kits. Patients nowadays are turning to smart self-monitoring in conditions like pregnancy, glucose level, and infectious diseases.
- OTC tests give consumers the possibility to perform diagnostic at their home without a healthcare facility visit. Fast results provide direct recommendations for further action, thus the number of appointments and travels is lessened. Such an advantage is especially attractive for the cases of a lack of time, or routine health check-ups.
- The prescription-based tests segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 6.3% over the forecast period. The growth of the segment is driven by increasing demand for accurate and clinically validated diagnostic solutions in hospitals and specialized care settings.
- Rising prevalence of chronic and infectious diseases necessitates timely physician-supervised testing for effective treatment. Technological advancements enabling faster, and more reliable results are enhancing their adoption. Additionally, supportive healthcare policies and insurance coverage are facilitating wider utilization of these tests.
Based on technology/platform, the rapid diagnostics market is segmented into flow-through, solid phase, lateral flow, agglutination assays, and other technology/platforms. The flow-through segment dominated the market in 2024, accounting for USD 13 billion and is anticipated to grow at a CAGR of 6.6% during the forecast period.
- Flow-through tests provide rapid detection of target analytes with extremely high sensitivity, delivering results in a matter of minutes. Their speed is, therefore, very well suited to emergency settings and the management of an outbreak. Such a fast turnaround time is, indeed, attractive to clinical laboratories and point-of-care applications.
- Many flow-through platforms are designed to enable multiplex testing that can detect multiple pathogens and biomarkers in one single assay. This ultimately leads to less sample volumes being required and reduces the duration of the testing, thus, magnifying the level of efficiency. The capability to multiplex infections panel is very useful in the field of infectious diseases and the practice of routine screening.
- The solid phase segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 6.3% over the forecast period. Solid-phase rapid diagnostics are driven by their high sensitivity and specificity, making them ideal for detecting low-abundance biomarkers. The simplicity of assay design and compatibility with automated platforms further enhance their adoption in clinical and point-of-care settings.
- The lateral flow segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 8% over the forecast period. The segment is driven by their ease of use, portability, and ability to deliver quick results without specialized equipment. Growing demand for point-of-care and home-based testing solutions further accelerates their adoption across healthcare and consumer markets.
- The agglutination assays segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 7.4% over the forecast period. The segmental growth is driven by their cost-effectiveness and ability to provide rapid qualitative and quantitative results. Their versatility in detecting a wide range of antigens and antibodies makes them highly suitable for clinical and field-based testing applications.
Based on distribution channel, the rapid diagnostics market is classified into brick and mortar and e-commerce. The brick and mortar segment dominated the market with a revenue share of 69.1% in 2024 and is expected to reach USD 46.8 billion within the forecast period.
- Physical healthcare facilities, clinics, and pharmacies are regarded as the first choice when it comes to diagnostic testing for accuracy and reliability. Patients, most of the time, prefer the professional administration of tests to assure the correct sample collection and the interpretation of the results. This trust is the main reason for the continued demand through the brick-and-mortar channels.
- Further, through brick-and-mortar installations, patients get direct access to the qualifications of healthcare professionals. The confidence and compliance of the patient are boosted by post-test counselling, the interpretation of results, and the prescription guidance. This kind of professional support is the reason for the use of rapid diagnostics in clinical settings.
- The e-commerce segment held a revenue of USD 10.9 billion in 2024, with projections indicating a steady expansion at 7.2% CAGR from 2025 to 2034. The segmental growth is driven by the growing consumer preference for convenient, home-delivered testing kits. Expanding online healthcare platforms and digital marketing strategies are further enhancing accessibility and market reach across urban and semi-urban regions.
Learn more about the key segments shaping this market
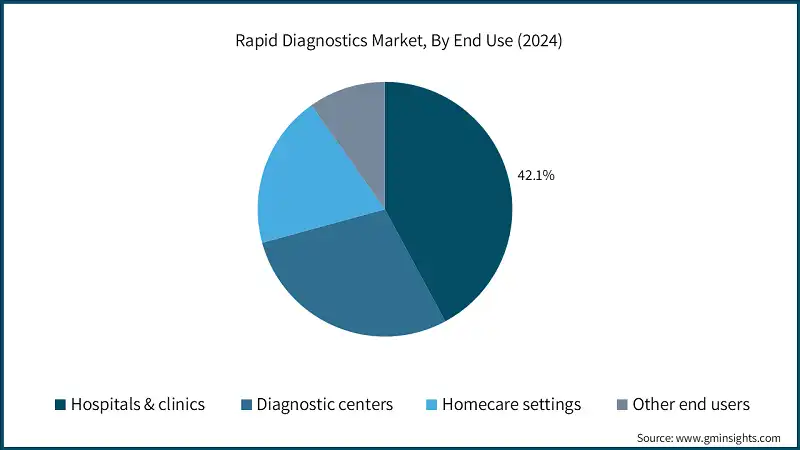
Based on end use, the rapid diagnostics market is classified into hospitals & clinics, diagnostic centers, homecare settings, and other end users. The hospitals & clinics segment dominated the market with a revenue share of 42.1% in 2024 and is expected to reach USD 29.9 billion within the forecast period.
- Hospitals and clinics prioritize quick diagnosis to make timely clinical decisions. Rapid diagnostic tests enable faster detection of infectious diseases, chronic conditions, and other critical health issues. This improves patient outcomes and reduces hospital stays, driving their adoption.
- Further, with the increased number of patients, hospitals require efficient diagnostic solutions that can assist in handling high workloads. Rapid tests considerably cut the waiting time compared to the conventional lab methods, thus workflow is considerably simplified. In that way, the efficiency equips institutions with the capacity to serve more patients without compromising the quality of care.
- The diagnostic centers segment held a revenue of USD 10.1 billion in 2024, with projections indicating a steady expansion at 6.2% CAGR from 2025 to 2034. The growth of this segment is largely fuelled by the demand for easy, on-site testing services that give quick and trustworthy results. The development of private medical institutions coupled with the heightened awareness of patients concerning preventive health check-ups are some of the market growth drivers.
- The homecare settings segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 7.5% over the forecast period. The segmental growth is driven by the growing preference for convenient, self-administered testing for chronic disease management and early detection. Increasing availability of user-friendly, portable diagnostic kits and remote monitoring technologies further accelerates adoption in residential care.
Looking for region specific data?
North America Rapid Diagnostics Market
North America dominated the market with the highest market share of 50.4% in 2024.
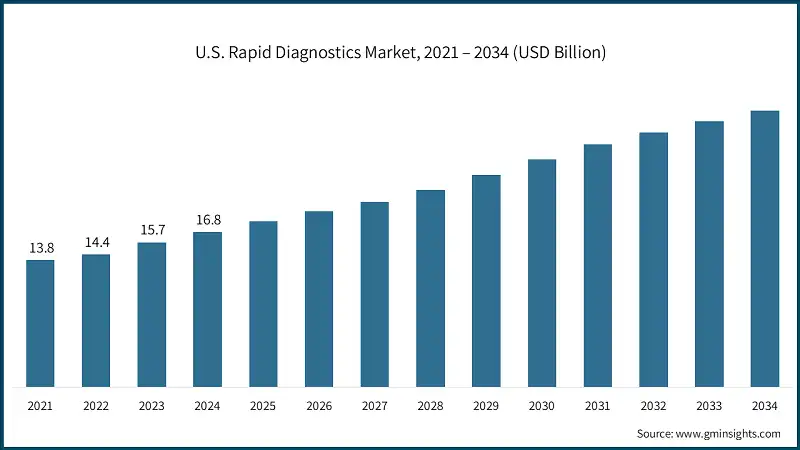
- The U.S. market was valued at USD 13.8 billion and USD 14.4 billion in 2021 and 2022, respectively. The market size reached USD 16.8 billion in 2024, growing from USD 15.7 billion in 2023, and is anticipated to grow at a CAGR of 5.8% between 2025 to 2034.
- North America boasts a healthcare system that is largely successful and which has the state-of-the-art diagnostic labs and hospital networks. This type of infrastructure serves as the basis for the rapid adoption of the innovative diagnostic technologies. The efficient lab networks also provide the possibility of large-scale testing and faster distribution of rapid diagnostic kits.
- In North America, there are frequent illnesses that are the main causes of the flu, RSV, and other infective respiratory diseases. The CDC announced that during the 2022-2023 flu season, the U.S. had an estimated number of flu hospitalizations ranging from 360,000 to 720,000 and deaths from 21,000 to 65,000. The CDC findings indicated that RSV hospitalizations elevated to 34.6 per 100,000 population in 2022. Public health authorities are investing in rapid diagnostic testing to better track and control these infections.
- The U.S. Department of Health and Human Services allocated USD 12.4 billion in 2023 for pandemic preparedness and biodefence programs, which includes funding for rapid diagnostic testing infrastructure. Programs focused on epidemic preparedness drive the implementation of rapid diagnostics across hospitals, clinics, and community settings.
Europe Rapid Diagnostics Market
Europe market accounted for USD 7.7 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- European governments make preventive healthcare a priority and early disease screening are part of the programs they organize. National health campaigns for infectious diseases, cancer, and chronic diseases encourage the use of rapid diagnostics. Subsidies and public funding for testing infrastructure are two forms of support that go beyond the mere market growth.
- Moreover, the region is home to one of the most rapidly growing elderly populations, which is a source of chronic and infectious diseases. The use of rapid diagnostic tests is the best option to meet health care needs, which enables a speedy detection and monitoring process. Hospitals and clinics install such devices to provide elderly patient care at a higher level and cut down on hospital visits.
- European diagnostic laboratories are investing in automation and modern diagnostic platforms. Integration of rapid tests into automated workflows reduces human error and turnaround time. This trend promotes wider adoption of rapid diagnostics across both private and public healthcare facilities.
Asia Pacific Rapid Diagnostics Market
The Asia Pacific market is anticipated to grow at the highest CAGR of 9.1% during the analysis timeframe.
- Countries across Asia Pacific, such as China, India, and Japan, are making massive investments in hospitals, clinics, and diagnostic laboratories. The expansion of healthcare facilities improves the availability of rapid diagnostic tests, particularly in the urban and semi-urban areas. Better infrastructure enables the quicker implementation of new diagnostic technologies.
- The region faces a significant burden of infectious diseases, including tuberculosis, malaria, dengue, and hepatitis. Rapid diagnostic tests are critical for timely detection and containment of outbreaks. This persistent healthcare challenge drives consistent demand for reliable and fast testing solutions.
- Several APAC governments actively endorse early detection and screening programs as part of their national healthcare campaigns. Such initiatives, when targeted at communicable diseases and maternal-child health, generate a large-scale demand for the use of rapid diagnostics. Public funding and subsidies are additional incentives that hospitals and community health centers receive to further their adoption.
Latin America Rapid Diagnostics Market
The Latin America market is experiencing robust growth over the analysis timeframe.
- In Latin America, the market is witnessing significant growth due to the rising prevalence of infectious diseases such as dengue, Zika, and COVID-19, which create a strong demand for timely detection. Increasing government initiatives and public health programs are enhancing access to diagnostic testing across urban and rural regions.
- Growing awareness among the population about early disease detection and preventive healthcare is further driving adoption. Expansion of private healthcare infrastructure and diagnostic laboratories is improving test availability and reach.
- Additionally, rising disposable incomes and the growing acceptance of home-based and point-of-care testing are contributing to market growth. The integration of digital health platforms and telemedicine services is also enabling faster reporting and remote patient management, supporting the overall market expansion.
Middle East and Africa Rapid Diagnostics Market
The Middle East and Africa market is experiencing robust growth over the analysis timeframe.
- The regional growth is driven by increasing government investments in healthcare infrastructure and initiatives to improve disease surveillance. The region faces a high burden of infectious diseases such as malaria, tuberculosis, and hepatitis, fueling demand for rapid testing solutions.
- Rising awareness about preventive healthcare and early diagnosis among the population is encouraging adoption. Expansion of private healthcare providers and diagnostic centers is enhancing accessibility to rapid tests. Technological advancements, including portable and easy-to-use diagnostic devices, are supporting their use in both urban and remote areas.
- Additionally, growing partnerships between local healthcare authorities and international diagnostic companies are facilitating market growth across the region.
Rapid Diagnostics Market Share
The global market is characterized by the presence of well-established global players and numerous regional companies focusing on innovative diagnostic solutions. Companies are heavily investing in research and development to develop faster, more accurate, and user-friendly testing platforms.
Key players include Abbott, Roche, Danaher, Quidel, and BIO-RAD, collectively accounting for 58.3% of the total market share. The market is highly competitive, with firms differentiating themselves through product portfolio expansion, strategic partnerships, and regional distribution networks.
Additionally, mergers, acquisitions, and collaborations with healthcare providers and government agencies are common strategies to enhance market presence and expand into emerging regions.
Players are also increasingly adopting digital integration and AI-driven diagnostics to offer value-added services and improve patient outcomes. Continuous innovation and regulatory approvals remain critical factors in maintaining a competitive edge and sustaining long-term growth in the market.
Rapid Diagnostics Market Companies
Few of the prominent players operating in the rapid diagnostics industry include:
- Abbott
- ACON
- Alfa Scientific
- Artron
- Becton, Dickinson and Company (BD)
- BIOMÉRIEUX
- BIO-RAD
- Danaher
- HOLOGIC
- Meridian Bioscience
- QIAGEN
- Quidel
- Roche
- Sight Diagnostics
- Thermo Fisher Scientific
- Trinity Biotech
- Abbott
- leading the rapid diagnostics market with a ~28.9% share in 2024, its extensive point-of-care testing portfolio, including BinaxNOW and i-STAT, offering rapid, reliable results across infectious diseases and chronic conditions. Its strong global distribution network ensures wide accessibility in both developed and emerging markets.
- Roche
Roche leverages advanced molecular and immunoassay technologies to provide highly accurate rapid diagnostics. Integration with digital health platforms and strong research and development capabilities enable continuous innovation and quick adaptation to emerging healthcare needs.
It’s expertise in high-quality immunology and molecular diagnostic assays, supporting both laboratory and point-of-care testing. The company emphasizes precision, reproducibility, and comprehensive solutions for infectious disease and clinical testing markets.
Rapid Diagnostics Industry News:
- In July 2025, PHASE Scientific announced that it had entered into an exclusive U.S. distribution agreement with Lumos Diagnostics for FebriDx, a rapid point-of-care test that diagnosed bacterial acute respiratory infections and differentiated them from non-bacterial causes in about 10 minutes using a single drop of blood. This agreement strengthened the rapid diagnostics market by expanding access to fast, accurate POC testing for respiratory infections.
- In May 2025, SEKISUI Diagnostics launched a rapid diagnostic tool, the OSOM RSV Test, for detecting RSV in healthcare settings. The test provided results from anterior nasal swabs in just 15 minutes. This launch is expected to boost the market by improving early detection, expanding point-of-care options, and enhancing the company’s infectious disease portfolio.
- In January 2024, QIAGEN launched two syndromic testing panels for its QIAstat-Dx instruments in India, including the Gastrointestinal Panel 2 and Meningitis/Encephalitis Panel, following the earlier authorization of the Respiratory SARS-CoV-2 Panel in 2020. This expansion strengthened the rapid diagnostics market by enabling faster, more accurate, and easier patient diagnosis in India.
- In July 2021, Abbott announced the launch of the Panbio COVID-19 Antigen Self-Test for detecting SARS-CoV-2 in adults and children, with or without symptoms. This launch supported the market by increasing accessibility for self-testing and enhancing early detection of COVID-19 cases.
The rapid diagnostics market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Billion from 2021 - 2034 for the following segments:
Market, By Product
- Infectious disease testing
- Respiratory infection testing products
- Influenza
- Hepatitis
- HIV
- Other infectious disease testing
- Pregnancy and fertility testing
- Hematology testing products
- Glucose monitoring
- Drug-of-abuse (DoA) testing products
- Cardiometabolic testing
- Coagulation testing products
- Cholesterol testing products
- Urinalysis testing products
- Tumor/cancer marker testing products
- Fecal occult testing products
- Other products
Market, By Purchase
- OTC tests
- Prescription-based tests
Market, By Technology/Platform
- Flow-through
- Solid phase
- Lateral flow
- Agglutination assays
- Other technology/platforms
Market, By Distribution Channel
- Brick and mortar
- E-commerce
Market, By End Use
- Hospitals & clinics
- Diagnostic centers
- Homecare settings
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Who are the key players in the rapid diagnostics market?
Key players include Abbott, ACON, Alfa Scientific, Artron, Becton, Dickinson and Company (BD), BIOMÉRIEUX, BIO-RAD, Danaher, and HOLOGIC.
Which region leads the rapid diagnostics market?
North America led the market in 2024, capturing 50.4% of the market share, driven by advanced healthcare infrastructure and widespread adoption of point-of-care testing solutions.
What are the upcoming trends in the rapid diagnostics industry?
Key trends include the shift towards molecular and genetic rapid tests, the rise of at-home and self-testing kits, integration with digital health and mobile apps, and the expansion into non-communicable diseases (NCDs).
What was the valuation of the OTC tests segment?
The OTC tests segment held a dominant position, generating USD 23.1 billion in 2024, with a projected CAGR of 7.1% during the forecast period.
What is the projected size of the rapid diagnostics market in 2025?
The market is expected to reach USD 38.1 billion in 2025.
How much revenue did the infectious disease testing segment generate?
The infectious disease testing segment accounting for 25.2% of the market share, and is projected to grow at a CAGR of 8.1% through 2034.
What is the market size of the rapid diagnostics in 2024?
The market size was USD 35.3 billion in 2024, with a CAGR of 6.8% expected through 2034, driven by the rising prevalence of infectious diseases, demand for point-of-care testing (POCT), and advancements in diagnostic technologies.
What is the projected value of the rapid diagnostics market by 2034?
The market is expected to reach USD 68.9 billion by 2034, fueled by technological innovations, integration with digital health platforms, and increasing healthcare investments.
Rapid Diagnostics Market Scope
Related Reports


