Summary
Table of Content

Point of Care Molecular Diagnostics Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Point of Care Molecular Diagnostics Market Size
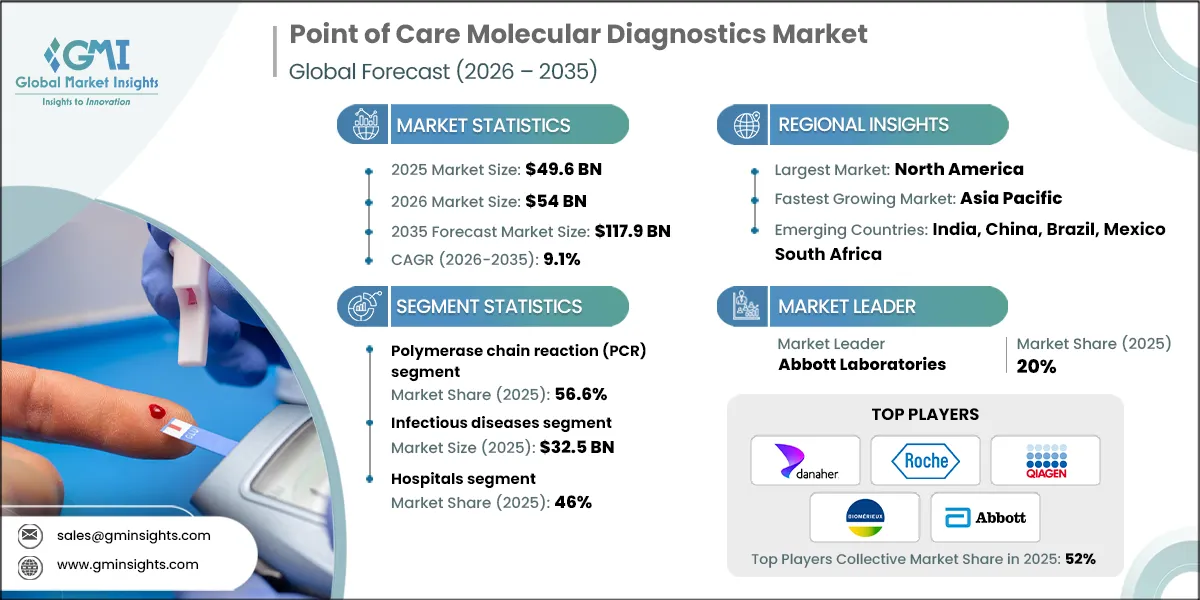
The global point of care molecular diagnostics market was valued at USD 49.6 billion in 2025 and is projected to grow from USD 54 billion in 2026 to USD 117.9 billion by 2035, expanding at a CAGR of 9.1%, according to the latest report published by Global Market Insights Inc.
To get key market trends
The steady growth of the market is driven by the increasing prevalence of chronic and infectious diseases, advancements in point-of-care molecular diagnostics technology, the growing need for rapid and accurate diagnostics, and the expanding geriatric population base.
The market grew from USD 38.6 billion in 2022 to USD 45.6 billion in 2024. The rising prevalence of chronic diseases, such as cardiovascular disorders, cancer, kidney diseases, and other noncommunicable conditions, is a major driver for the point-of-care molecular diagnostics market. For instance, according to the World Health Organization (WHO), noncommunicable diseases (NCDs) account for 41 million deaths annually, representing 74% of global mortality.
Each year, 17 million people die from NCDs before the age of 70, with 86% of these premature deaths occurring in low- and middle-income countries. These statistics underscore the growing burden of NCDs, including cardiovascular diseases, cancer, and renal disorders, which significantly fuels the demand for rapid, decentralized molecular testing solutions. The ability of point-of-care molecular diagnostics to deliver accurate, real-time results at the patient’s bedside or in resource-limited settings makes it an essential tool for early detection, treatment monitoring, and improving clinical outcomes in chronic disease management.
Additionally, the growing geriatric population base is a significant driver for the point-of-care molecular diagnostics market. As global demographics shift toward older age groups, the prevalence of age-related conditions such as cardiovascular diseases, cancer, kidney disorders, and metabolic complications continues to rise, creating greater demand for rapid and accurate molecular testing solutions. For instance, according to the World Health Organization (WHO), the global population aged 60 years and over is projected to increase from 1 billion in 2020 to 1.4 billion by 2030, eventually doubling to 2.1 billion by 2050.
Furthermore, the number of individuals aged 80 years and older is expected to triple from 2020 to 2050, reaching 426 million. Thus, this demographic shift underscores the growing need for point-of-care molecular diagnostics that enable timely detection and monitoring of chronic and age-related conditions, supporting improved clinical decision-making and patient outcomes.
Point-of-care molecular diagnostics refers to rapid, portable diagnostic solutions that use molecular biology techniques such as PCR or isothermal amplification to detect genetic, genomic, or pathogen-specific markers directly at or near the patient’s location. These tests deliver highly accurate and sensitive results within minutes to hours, enabling immediate clinical decisions without the need for centralized laboratory processing.
Point of Care Molecular Diagnostics Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2025 |
| Market Size in 2025 | USD 49.6 Billion |
| Market Size in 2026 | USD 54 Billion |
| Forecast Period 2026-2035 CAGR | 9.1% |
| Market Size in 2035 | USD 117.9 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of chronic and infectious diseases | Driving demand for rapid molecular testing at the point of care, enabling early detection and improved treatment outcomes. |
| Technological advancements in point of care molecular diagnostics | Innovations such as portable PCR systems, isothermal amplification, and integrated digital platforms are enhancing test accuracy and speed, expanding adoption in emergency and decentralized settings. |
| Growing need for rapid and accurate diagnostics | The ability to deliver real-time molecular results at the patient’s location reduces reliance on centralized labs, accelerates clinical decision-making, and improves patient management. |
| Rising geriatric population base | Increasing elderly population with higher susceptibility to chronic and infectious diseases is fueling demand for point-of-care molecular diagnostics for timely screening and monitoring. |
| Pitfalls & Challenges | Impact |
| High cost of POC molecular devices | Advanced molecular platforms and consumables remain expensive, limiting adoption in resource-constrained settings and smaller healthcare facilities. |
| Stringent regulatory scenario | Complex approval processes and compliance requirements delay product launches and increase costs, creating barriers for new entrants and slowing innovation. |
| Opportunities: | Impact |
| Growing healthcare expansion in emerging economies | Rising investments in healthcare infrastructure and demand for decentralized testing in rural and remote areas create significant growth potential for point-of-care molecular diagnostics. |
| Market Leaders (2025) | |
| Market Leader |
20% market share |
| Top Players |
52% market share |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | India, China, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Point of Care Molecular Diagnostics Market Trends
Technological innovations in point-of-care molecular diagnostic platforms are driving market growth by improving speed, accuracy, and ease of use. These advancements have transformed decentralized testing, enabling rapid molecular analysis in diverse clinical settings such as emergency medicine, critical care, and resource-limited environments, while enhancing functionality and reliability.
Modern POC molecular systems have evolved from complex laboratory setups to compact, user-friendly devices that deliver fast and precise results. Many of these platforms integrate seamlessly with digital interfaces, allowing clinicians to perform nucleic acid amplification and pathogen detection at the patient’s location. This significantly reduces turnaround time and improves treatment decisions.
- For instance, Abbott ID NOW, a rapid molecular testing platform, delivers accurate results for infectious diseases within minutes using isothermal nucleic acid amplification technology. Built for urgent care and decentralized settings, it combines speed and simplicity to support timely clinical interventions.
- In addition, advances in assay design and microfluidics have greatly improved sensitivity and specificity in POC molecular diagnostics. Modern systems now support multiplex testing, enabling simultaneous detection of multiple pathogens or genetic markers. This expands their use from single-target assays to comprehensive diagnostic applications.
- For example, Cepheid GeneXpert offers cartridge-based molecular testing with high sensitivity and supports multiple assays for infectious diseases, oncology, and genetic conditions, making it a versatile solution for point-of-care diagnostics.
- Furthermore, POC molecular platforms now provide seamless integration with electronic health record (EHR) systems and cloud-based platforms, enabling clinicians to upload test results directly into patient records. This connectivity improves workflow efficiency, supports remote consultations, and continues to drive market adoption globally.
Point of Care Molecular Diagnostics Market Analysis
Learn more about the key segments shaping this market
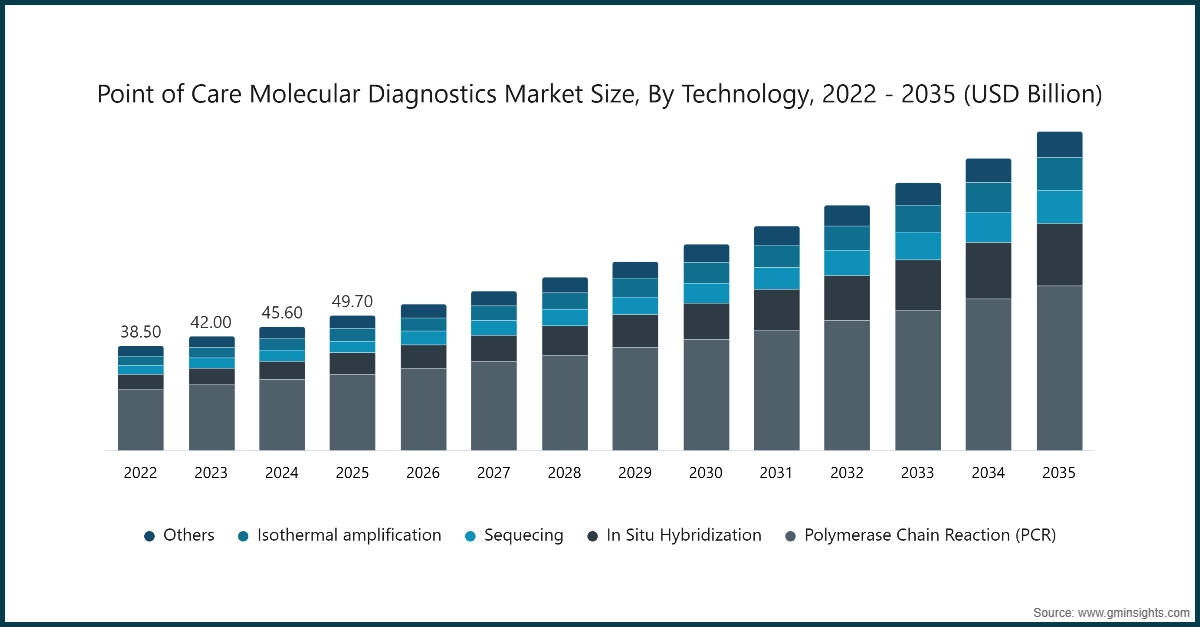
Based on technology, the point of care molecular diagnostics market is segmented into polymerase chain reaction (PCR), in situ hybridization, sequencing, isothermal amplification, and other technologies. The polymerase chain reaction (PCR) segment was valued at USD 28.1 billion in 2025 and held a significant market share of 56.6%.
- PCR-based point-of-care (POC) devices are essential components of molecular diagnostics, enabling rapid and accurate detection of pathogens, genetic markers, and infectious diseases. These systems provide clinicians with actionable results within minutes, supporting timely treatment decisions and improving patient outcomes in emergency, outpatient, and resource-limited settings.
- Modern POC PCR platforms are highly compact and user-friendly, featuring cartridge-based designs that minimize manual steps. With integrated sample preparation, real-time amplification, and automated result interpretation, these devices deliver laboratory-grade accuracy in decentralized environments.
- For example, Cepheid’s GeneXpert Xpress system offers a fully automated PCR solution for point-of-care use. Its intuitive interface and single-use cartridges streamline testing for respiratory pathogens, including SARS-CoV-2 and influenza, delivering results in under 30 minutes with minimal operator intervention.
- Additionally, the growing need for rapid infectious disease diagnostics, the rising prevalence of antimicrobial resistance, and global initiatives to strengthen decentralized healthcare are driving the adoption of advanced POC PCR systems. These innovations are fueling market growth by improving accessibility, reducing turnaround times, and enabling high-quality molecular testing in diverse clinical environments.
Based on application, the point of care molecular diagnostics market is segmented into infectious diseases, oncology, hematology, and other applications. The infectious diseases segment was valued at USD 32.5 billion in 2025.
- The rising prevalence of infectious diseases, such as influenza, respiratory syncytial virus (RSV), tuberculosis (TB), HIV, gonorrhea, and other pathogens, is a major driver of market growth.
- For example, according to the World Health Organization, tuberculosis caused 1.23 million deaths globally in 2024, including 150,000 among people with HIV. TB remains the leading cause of death from a single infectious agent and ranks among the top 10 causes of death worldwide.
- Point-of-care (POC) molecular diagnostics enable rapid and accurate detection of infectious pathogens near the patient, delivering results within minutes in decentralized settings such as clinics and emergency care. This approach eliminates delays associated with traditional laboratory PCR testing, supporting timely treatment and effective infection control.
- Furthermore, the integration of POC molecular diagnostics in infectious disease management enhances patient safety, reduces transmission risk, and minimizes reliance on costly hospital-based testing. Its ability to provide accurate results at the point of care improves workflow efficiency and accelerates treatment initiation.
Learn more about the key segments shaping this market
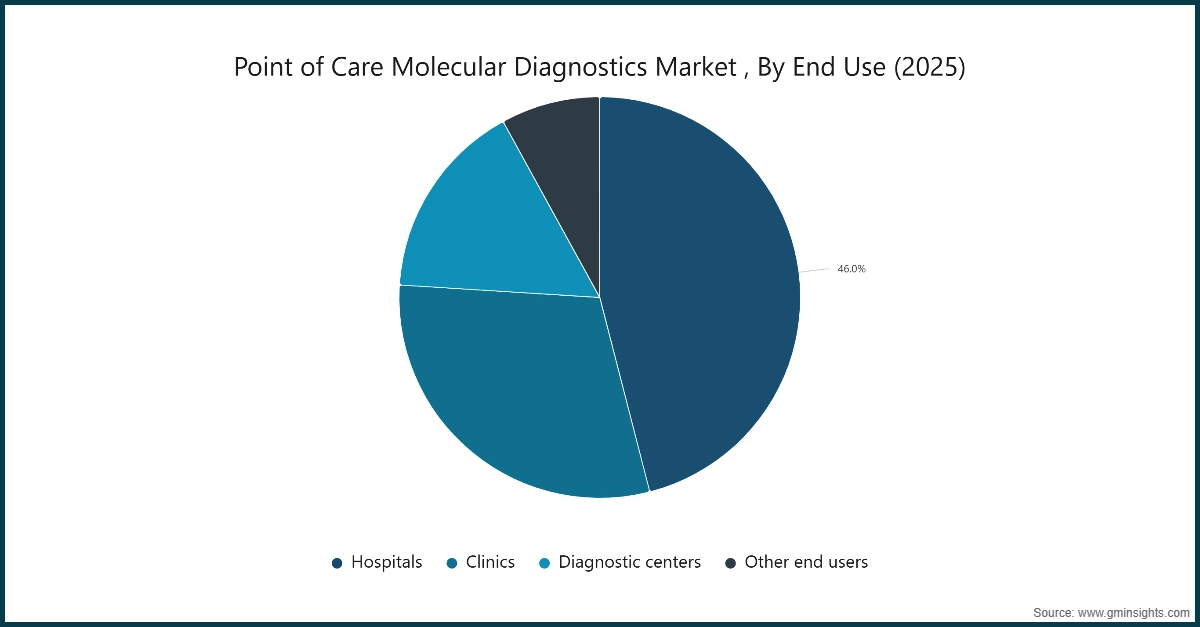
Based on end use, the point of care molecular diagnostics market is segmented into hospitals, clinics, diagnostic centers, and other end users. The hospitals segment held a significant market share of 46% in 2025.
- Hospitals represent the largest end user segment in the point-of-care (POC) molecular diagnostics market due to their critical need for rapid and accurate testing in high-acuity environments such as emergency departments, ICUs, operating rooms, and inpatient wards. These settings require immediate results to guide treatment decisions and prevent disease transmission.
- POC molecular diagnostic systems in hospitals are primarily used for detecting infectious diseases such as influenza, RSV, tuberculosis, HIV, and other pathogens. Their ability to deliver results within 15–30 minutes allows clinicians to initiate targeted therapies without delays associated with centralized laboratory workflows, improving patient outcomes and reducing hospital-acquired infections.
- The growing burden of infectious diseases, rising ICU admissions, and increased surgical procedures are driving hospitals to adopt advanced POC molecular platforms. Modern solutions feature integrated sample preparation, automated PCR amplification, and connectivity tools for seamless integration with hospital information systems and electronic health records (EHRs), ensuring real-time data sharing and collaborative care.
- These innovations enhance operational efficiency, reduce diagnostic turnaround times, and support infection control protocols, making POC molecular diagnostics indispensable in hospital settings and driving market growth.
North America Point of Care Molecular Diagnostics Market
Looking for region specific data?
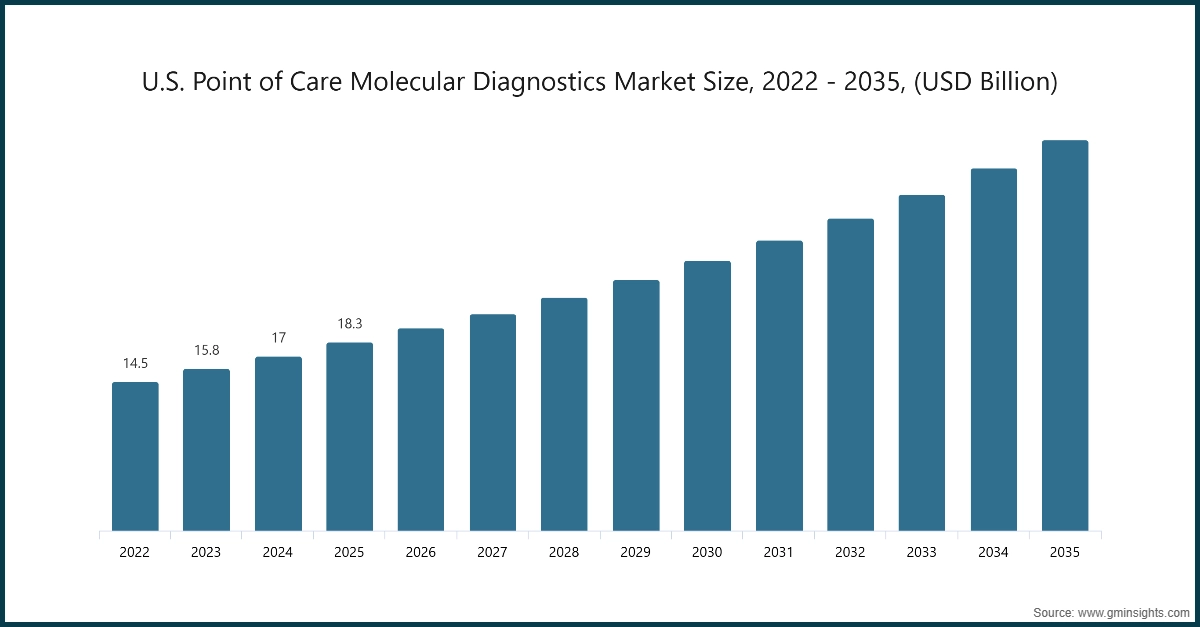
The North America region accounted for 39.1% of the point of care molecular diagnostics market in 2025. The market in North America is experiencing robust expansion, driven by the region’s advanced healthcare infrastructure and technological advancements.
- The U.S. point of care molecular diagnostics market was valued at USD 14.5 billion and USD 15.8 billion in 2022 and 2023, respectively. In 2025 the market size increased to USD 18.3 billion from USD 17 billion in 2024.
- The growth of the U.S. point-of-care (POC) molecular diagnostics market is primarily driven by the rising prevalence of infectious diseases and chronic conditions. This trend has created a strong demand for rapid and accurate near-patient testing solutions.
- For example, according to the National Cancer Institute, there were an estimated 18.1 million cancer survivors in the U.S. as of January 2022. This number is projected to increase to 26 million by 2040.
- The U.S. leads in the adoption and innovation of POC molecular diagnostics due to its advanced healthcare infrastructure, high diagnostic testing volumes, and continuous investment in decentralized testing technologies. Hospitals and diagnostic centers collaborate with leading manufacturers to integrate automated PCR systems, AI-driven interpretation, and connectivity features. These advancements enable seamless EHR integration and real-time data sharing, ensuring improved patient care and operational efficiency.
Europe Point of Care Molecular Diagnostics Market
Europe point of care molecular diagnostics industry accounted for USD 13.9 billion in 2025 and is anticipated to show lucrative growth over the forecast period.
- The European point-of-care (POC) molecular diagnostics industry is positioned for strong growth, driven by advanced healthcare infrastructure and increasing demand for rapid, decentralized testing solutions across hospitals and clinics. The need for accurate, near-patient diagnostics in emergency, critical care, and outpatient settings is accelerating adoption across the region.
- European governments are making significant investments in healthcare modernization, supporting the integration of advanced molecular diagnostic technologies. For instance, EU member states have allocated over USD 11.7 billion (EUR 10 billion) for healthcare infrastructure upgrades between 2021 and 2027. This investment creates substantial opportunities for POC molecular platforms in infectious disease management and routine clinical workflows.
Germany's point of care molecular diagnostics market is projected to experience steady growth between 2026 and 2035.
- The growing aging population in Germany is a key driver for the adoption of point-of-care (POC) molecular diagnostics. According to Statista, in 2024, individuals aged 40–59 represent the largest age group at approximately 22.3 million, while those aged 65 and older account for around 19 million. This demographic trend increases the prevalence of infectious and chronic conditions, fueling demand for rapid, near-patient molecular testing.
- Germany is emerging as a significant market for POC molecular diagnostics, supported by its advanced healthcare infrastructure, strong clinical expertise, and increasing adoption of decentralized testing solutions in emergency, critical care, and outpatient settings.
- Public-private initiatives and investments in hospital modernization further promote the integration of innovative POC molecular platforms, including automated PCR systems, AI-assisted interpretation, and connectivity features for seamless data sharing. These advancements enhance diagnostic capabilities for conditions such as respiratory infections, tuberculosis, and sexually transmitted diseases, ensuring timely treatment and improved patient outcomes.
Asia Pacific Point of Care Molecular Diagnostics Market
The Asia Pacific region is projected to show a lucrative growth of about 11.2% during the forecast period.
- The point-of-care (POC) molecular diagnostics industry in the Asia-Pacific region is witnessing rapid growth, driven by the rising prevalence of infectious diseases, increasing focus on early detection, and advancements in portable molecular testing technologies.
- Countries such as China, India, and Japan are accelerating the adoption of POC molecular solutions due to growing demand for fast, accurate diagnostic capabilities and improved access to decentralized healthcare services. These solutions are particularly critical in emergency care, rural health programs, and high-volume outpatient settings.
- Additionally, the expansion of hospitals, diagnostic centers, and emergency facilities, combined with government-led initiatives to strengthen point-of-care testing infrastructure, is significantly boosting market growth. Supportive policies and investments aimed at improving infectious disease management and pandemic preparedness further enhance the integration of POC molecular platforms across the region.
China point of care molecular diagnostics market is poised to witness lucrative growth between 2026 - 2035.
- China's aging population is increasingly driving the need for regular health monitoring.
- For example, according to WHO estimates (2019), approximately 254 million people aged 65 and above were living in China. This number is projected to rise significantly, with around 402 million individuals expected to be over 60 by 2040. The growing burden of respiratory infections, tuberculosis, and other communicable diseases highlights the importance of decentralized testing capabilities.
- POC molecular diagnostic platforms in China provide fast and reliable results within minutes, supporting early intervention and infection control in hospitals, emergency care, and rural health settings. Government initiatives to strengthen healthcare infrastructure, combined with investments in advanced technologies such as automated PCR systems, AI-driven interpretation, and cloud-based connectivity, are accelerating adoption and driving substantial market growth.
Latin America Point of Care Molecular Diagnostics Market
Brazil is experiencing significant growth in the point of care molecular diagnostics industry.
- The growth of the point-of-care (POC) molecular diagnostics industry in Brazil is strongly driven by technological advancements and the introduction of innovative portable PCR-based testing devices, along with the rising prevalence of infectious diseases and chronic health conditions in the country.
- Healthcare facilities and emergency care settings in Brazil are increasingly adopting POC molecular diagnostic systems that deliver faster and highly accurate results compared to traditional laboratory-based methods. These solutions enable timely detection and treatment initiation, improving patient outcomes and infection control.
Middle East and Africa Point of Care Molecular Diagnostics Market
- Saudi Arabia is steadily increasing its research and development expenditure in healthcare, with significant investments from both government and private sectors aimed at modernizing infrastructure and fostering innovation in diagnostic technologies. These efforts are creating a strong foundation for the adoption of advanced point-of-care (POC) molecular diagnostic solutions.
- POC molecular diagnostics provide rapid and accurate detection of infectious diseases directly at the patient’s location, enabling timely clinical decisions in emergency, critical care, and outpatient settings. This capability aligns with Saudi Arabia’s healthcare modernization goals and its focus on improving patient outcomes through decentralized testing.
Point of Care Molecular Diagnostics Market Share
The top five players in the point-of-care (POC) molecular diagnostics industry, including Abbott Laboratories, Danaher Corporation, F. Hoffmann-La Roche, QIAGEN, and BioMérieux SA, collectively hold a 52% share of the global market. These companies continue to strengthen their positions through innovation, regulatory compliance, and strategic collaborations. Leading players are heavily investing in research and development to introduce next-generation POC molecular platforms with enhanced speed, portability, and AI-driven interpretation capabilities.
- Abbott maintains a strong position in the POC molecular diagnostics segment through its flagship ID NOW platform, which provides rapid molecular testing for respiratory pathogens, including influenza and SARS-CoV-2, delivering results in under 15 minutes. The company focuses on expanding its test menu, improving connectivity features for seamless integration with electronic health records (EHRs), and leveraging cloud-based platforms to enable real-time data sharing and telehealth support.
- Manufacturers are adopting value-based pricing strategies to penetrate cost-sensitive markets, particularly in emerging economies. Leading players are addressing clinical needs by launching compact, cartridge-based molecular systems and AI-assisted interpretation tools, ensuring reliability and convenience for both hospital and outpatient environments. These advancements aim to enhance diagnostic accuracy and accessibility, supporting better management of infectious diseases and chronic conditions.
- Emerging trends in the POC molecular diagnostics industry include integration with telehealth platforms for remote consultations, AI-driven analytics for predictive diagnostics, and cloud-enabled connectivity for real-time reporting. There is also a growing focus on sustainability, with manufacturers developing energy-efficient devices and eco-friendly consumables to reduce environmental impact.
Point of Care Molecular Diagnostics Market Companies
Few of the prominent players operating in the point of care molecular diagnostics industry include:
- Abbott Laboratories
- Abaxis
- Akonni Biosystems
- Bayer
- BioMérieux
- Bio-Rad Laboratories
- Danaher Corporation
- F. Hoffmann-La Roche
- Sysmex Corporation
- Mesa Biotech
- Nova Biomedical
- OraSure Technologies
- QIAGEN
- QuidelOrtho
- VIRCELL S.L
Abbott leads the POC molecular diagnostics market with its ID NOW platform, which provides rapid PCR testing for respiratory pathogens in under 15 minutes. The company focuses on expanding its test menus, improving EHR connectivity, and enabling real-time reporting through cloud integration. These advancements make its solutions well-suited for hospitals, urgent care centers, and decentralized settings.
Roche Diagnostics holds a strong position in the POC molecular diagnostics industry with its cobas Liat system, which delivers highly accurate PCR testing for infectious diseases at the point of care. Roche invests significantly in automation, connectivity, and AI-driven interpretation to enhance diagnostic speed and reliability.
Cepheid, a subsidiary of Danaher, is recognized as a pioneer in POC molecular diagnostics with its GeneXpert Xpress platform. This platform provides rapid PCR-based testing for respiratory infections, tuberculosis, and sexually transmitted diseases. The company emphasizes cartridge-based simplicity, automated workflows, and cloud-enabled connectivity to facilitate real-time data sharing.
Point of Care Molecular Diagnostics Industry News:
- In January 2025, BioMérieux entered into an agreement to acquire SpinChip Diagnostics ASA, a Norwegian diagnostics company. This acquisition strengthened bioMérieux’s position in the point-of-care molecular diagnostics industry by expanding its technology portfolio and accelerating innovation in rapid testing solutions, enhancing its competitive edge in decentralized diagnostics.
- In March 2020, Abbott received FDA Emergency Use Authorization (EUA) for its ID NOW COVID-19 test, the fastest molecular point-of-care test at the time, delivering positive results in as little as five minutes and negatives in 13 minutes. This breakthrough enabled rapid testing in diverse healthcare settings, including urgent care clinics and emergency departments, significantly strengthening Abbott’s market leadership in POC molecular diagnostics and driving widespread adoption during the pandemic.
The point of care molecular diagnostics market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2022 - 2035 for the following segments:
Market, By Technology
- Polymerase chain reaction (PCR)
- In situ hybridization
- Sequencing
- Isothermal amplification
- Other technologies
Market, By Application
- Infectious diseases
- Flu
- Respiratory syncytial virus (RSV)
- Tuberculosis (TB)
- HIV
- Gonorrhea
- Chlamydia
- Hepatitis C
- Hepatitis B
- COVID-19
- Other infectious diseases
- Oncology
- Hematology
- Other applications
Market, By End Use
- Hospitals
- Clinics
- Diagnostic centers
- Other end users
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- Saudi Arabia
- South Africa
- UAE
Frequently Asked Question(FAQ) :
What are the upcoming trends in the point-of-care molecular diagnostics market?
Trends include advancements in assay design, microfluidics for multiplex testing, integration with EHR systems, cloud-based platforms, and the development of compact, user-friendly diagnostic devices.
Who are the key players in the point-of-care molecular diagnostics industry?
Key players include Abbott Laboratories, Abaxis, Akonni Biosystems, Bayer, BioMérieux, Bio-Rad Laboratories, Danaher Corporation, Mesa Biotech, Nova Biomedical, OraSure Technologies, QIAGEN, QuidelOrtho, and VIRCELL S.L.
What is the growth outlook for the hospitals segment?
The hospitals segment held a 46% market share in 2025, driven by the critical need for rapid and accurate testing in high-acuity environments like emergency departments and ICUs.
Which region leads the point-of-care molecular diagnostics sector?
North America leads the market with a 39.1% share in 2025, supported by advanced healthcare infrastructure and technological advancements.
What is the expected size of the point-of-care molecular diagnostics industry in 2026?
The market size is projected to reach USD 54 billion in 2026.
How much revenue did the polymerase chain reaction (PCR) segment generate in 2025?
The PCR segment generated approximately USD 28.1 billion in 2025, accounting for a significant 56.6% market share.
What was the valuation of the infectious diseases segment in 2025?
The infectious diseases segment was valued at USD 32.5 billion in 2025, led by the rising prevalence of diseases such as influenza, RSV, TB, HIV, and gonorrhea.
What is the projected value of the point-of-care molecular diagnostics market by 2035?
The market is poised to reach USD 117.9 billion by 2035, expanding at a CAGR of 9.1%, fueled by technological innovations and the demand for rapid and accurate diagnostics.
What was the market size of the point-of-care molecular diagnostics in 2025?
The market size was USD 49.6 billion in 2025, driven by the increasing prevalence of chronic and infectious diseases, advancements in diagnostics technology, and the growing geriatric population.
Point of Care Molecular Diagnostics Market Scope
Related Reports


