Summary
Table of Content

Molecular Diagnostics Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
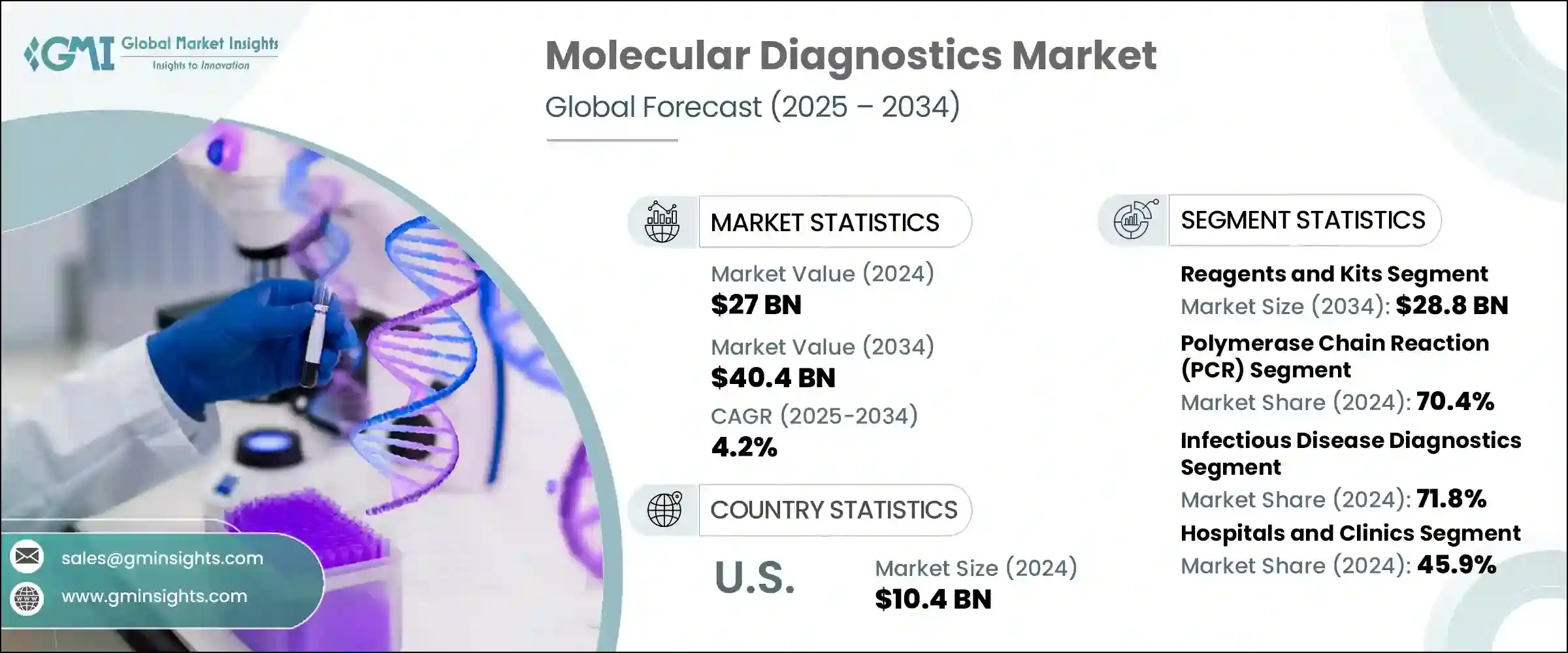
Molecular Diagnostics Market Size
The global molecular diagnostics market was valued at USD 27 billion in 2024 and is projected to grow from USD 27.9 billion in 2025 to USD 40.4 billion by 2034, expanding at a CAGR of 4.2%.
To get key market trends
This growth is primarily attributed to various stimulating factors, including the rising prevalence of infectious diseases, technological advancements in molecular diagnostics, increasing global awareness of early disease diagnosis, the escalating demand for POC diagnostics, and the growing geriatric population base. Molecular diagnostics is a diagnostic technique used to analyze biological markers in the proteome and genome, specifically RNA, DNA, and protein, to detect and monitor various diseases. Major companies in the industry include Abbott Laboratories, F. Hoffmann-La Roche, Danaher Corporation, Siemens Healthineers, and Thermo Fisher Scientific.
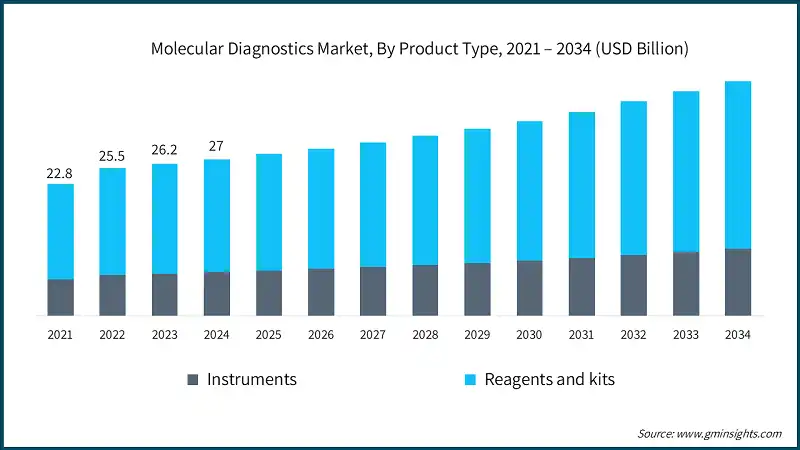
The market has increased from USD 22.8 billion in 2021 to USD 26.2 billion in 2023. This growth has been attributed to the widespread prevalence of COVID-19, which has created a huge demand for efficient and more accurate rapid diagnostic testing. One of the most widespread molecular diagnostic technologies used during this period has been the Reverse Transcription Polymerase Chain Reaction (RT-PCR), which has served as the gold standard for the detection of the SARS-CoV-2 virus due to its advanced features, such as high specificity and sensitivity.
The increasing prevalence of infectious diseases, including COVID-19, influenza, respiratory syncytial virus (RSV), tuberculosis, HIV, hepatitis C, and hepatitis B, has significantly driven the growth of the molecular diagnostics market. According to the World Health Organization (WHO), as of 2023, approximately 39.9 million people globally have been living with HIV, with around 630,000 deaths reported due to AIDS-related illnesses. Additionally, approximately 230 million individuals across 103 countries have undergone HIV diagnostic testing, reflecting strong global efforts toward disease prevention. These trends underscore the rising demand for effective and reliable molecular diagnostic instruments and kits for early disease detection, thereby supporting market growth.
Additionally, the growing geriatric population worldwide has significantly contributed to the expansion of the molecular diagnostics market. According to the World Health Organization (WHO), the global population aged 60 years and above is projected to increase from 1.1 billion in 2023 to 1.4 billion by 2030. In China, approximately 254 million individuals aged 65 and above were recorded in 2019, with projections indicating this figure will rise to 402 million people over the age of 60 by 2040. These trends underscore the importance of regular health screenings, as older populations are more susceptible to chronic diseases. Consequently, the demand for advanced, user-friendly molecular diagnostic solutions is expected to grow as healthcare systems adapt to meet the needs of aging populations.
Molecular diagnostics refers to a diagnostic method that examines biological markers in the genome and proteome, including RNA, DNA, and proteins, to identify and monitor various diseases. This technique is widely utilized for diagnosing genetic disorders, infectious diseases, and cancer due to its high effectiveness and precision.
Molecular Diagnostics Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 27 Billion |
| Forecast Period 2025 – 2034 CAGR | 4.2% |
| Market Size in 2034 | USD 40.4 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Technological advances and increasing awareness towards early disease diagnosis globally | Improved diagnostic accuracy and accessibility are driving widespread adoption, enabling earlier and more effective treatment interventions. |
| Escalating demand for POC diagnostics | POC diagnostics support rapid testing and decision-making, especially in remote or resource-limited settings, enhancing patient outcomes. |
| Increasing geriatric population base | Aging populations require frequent monitoring and early diagnosis, increasing demand for reliable and accessible diagnostic solutions. |
| Increasing number of R&D initiatives | Continuous innovation is expanding diagnostic capabilities, improving test sensitivity and specificity, and fostering market growth. |
| Rising incidences of infectious diseases | Global outbreaks and endemic diseases are accelerating the need for fast, accurate diagnostics to contain and manage infections. |
| Pitfalls & Challenges | Impact |
| High cost of molecular diagnostic tests | Elevated costs restrict access in low-income regions and challenge healthcare systems to balance quality with affordability. |
| Lack of skilled personnel | Shortage of trained professionals limits the deployment and effective use of advanced diagnostic technologies, especially in emerging markets. |
| Opportunities: | Impact |
| Expansion in emerging markets | Growing healthcare infrastructure and awareness in developing regions offer significant potential for market penetration and revenue growth. |
| Integration with artificial intelligence (AI) | AI enhances diagnostic speed, accuracy, and predictive power, streamlining workflows and enabling personalized medicine. |
| Market Leaders (2024) | Market Leader |
|
| Top Players |
Collective Market Share in 2024 is 34.6% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Countries | India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Molecular Diagnostics Market Trends
- The global market is evolving rapidly, driven by both macro- and micro-level trends. At the macro level, technological advancements are a key driver of market growth. Leading companies are introducing user-friendly and compact molecular diagnostic devices capable of performing complex tests outside traditional laboratory settings, thereby accelerating market expansion.
- For example, Huwel Lifesciences’ RT-PCR system illustrates this trend. This portable and compact molecular diagnostic device is designed for research, diagnostics, and field testing. It supports up to 16 samples with fast ramp rates for time-sensitive workflows. Additionally, the system is maintenance-free and features USB compatibility for seamless data transfer.
- The increasing prevalence of cancer is another significant factor driving the demand for advanced molecular diagnostic devices and kits. According to the American Cancer Society, approximately 2 million new cancer cases and around 611,720 cancer-related deaths were projected in 2024, highlighting the need for effective molecular diagnostic tools.
- Furthermore, the growing adoption of point-of-care molecular diagnostics is revolutionizing healthcare delivery in developing regions by bringing diagnostic capabilities closer to patients, particularly in remote and resource-limited areas. Point-of-care testing, estimated to reach USD 78.3 billion by 2032, addresses challenges such as inadequate infrastructure, limited medical access, and the rising burden of infectious diseases. This enables timely diagnosis and treatment, improving patient management.
- For instance, 28% of Australia’s population resides in rural areas, while in India, 86% of medical consultations involve rural residents, many of whom travel over 100 kilometers for healthcare services. Molecular point-of-care testing enhances disease management in such regions, leading to improved health outcomes and further driving the growth of the market.
Molecular Diagnostics Market Analysis
Learn more about the key segments shaping this market
The global market was valued at USD 22.8 billion in 2021. The market size reached USD 26.2 billion in 2023, from USD 25.5 billion in 2022.
Based on the product type, the market is segmented into instruments and reagents and kits. The reagents and kits segment led this market in 2024, accounting for the highest market share because of its frequent repeat purchases accelerated by its high-throughput workflow and routine diagnostic testing. This segment was valued at USD 19.4 billion in 2024 and is projected to reach USD 28.8 billion by 2034, growing at a CAGR of 4.1%. This growth is largely attributed to the rising prevalence of chronic disease conditions and rising aging population across the globe. In comparison, the instruments segment, valued at USD 7.6 billion in 2024, is expected to grow to USD 11.5 billion by 2034, with a CAGR of 4.4%.
- The growth of the reagents and kits segment is primarily driven by their increasing utilization in research and clinical applications.
- Unlike instruments, reagents and kits are purchased regularly and in bulk quantities. This recurring demand is particularly evident in clinical laboratories and hospitals, where routine diagnostic testing is conducted due to the rising prevalence of infectious diseases.
- Infectious diseases, such as tuberculosis, significantly contribute to the growing need for advanced reagents and kits for early and accurate disease diagnosis.
- For example, according to the World Health Organization, approximately 10.8 million people were affected by tuberculosis in 2023, up from 10.7 million in 2022. This increase in prevalence directly correlates with higher testing volumes, driving the demand for reagents and kits.
- Additionally, standardized reagents and kits ensure accurate and efficient results. Their cost-effectiveness, reliability, and ability to deliver consistent outcomes are expected to further support the growth of this segment.
Based on technology, the molecular diagnostics market is segmented into polymerase chain reaction (PCR), hybridization, sequencing, isothermal nucleic acid amplification technology (INAAT), microarrays, and other technologies. The polymerase chain reaction (PCR) segment accounted for the highest market share of 70.4% in 2024. In comparison, the hybridization segment, valued at USD 2.8 billion in 2024, is expected to grow to USD 4 billion by 2034, with a CAGR of 4%.
- PCR remains one of the significant technologies in the field of molecular diagnostics due to its ability to amplify DNA and RNA precisely at a rapid pace, which makes it ideal for detecting various pathogens responsible for infectious diseases, genetic mutations, and other biomarkers.
- Moreover, PCR’s high sensitivity enables the detection of even a minute quantity of nucleic acid, which is crucial for early disease detection and monitoring, further propelling its adoption in various disease detection applications.
- Additionally, next-generation sequencing (NGS) technologies have several advantages and provide new diagnostic possibilities. Some significant advantages of NGS include higher sequencing capacity, higher diagnostic sensitivity, workflow miniaturization, and cost benefits, thereby accelerating segmental growth. Additionally, wide application in the field of molecular oncology is further expected to help in segmental growth.
Based on application, the molecular diagnostics market is segmented into infectious disease diagnostics, genetic disease testing, oncology testing, and other applications. The infectious disease diagnostics market is further bifurcated into, COVID-19, flu, respiratory syncytial virus (RSV), tuberculosis, CT/NG, HIV, Hepatitis C, Hepatitis B, and other infectious disease diagnostics. The infectious disease diagnostics segment accounted for the highest market share of 71.8% in 2024. In comparison, the oncology testing segment, valued at USD 2.1 billion in 2024, is expected to grow to USD 3.2 billion by 2034.
- The increasing global prevalence of cancer is a key driver of growth in this segment.
- According to estimates from the Cancer Atlas, the global incidence of cancer is expected to rise by 60% by 2040, reaching approximately 29.4 million new cases, compared to 18.1 million cases reported in 2018.
- This growing cancer burden, driven by factors such as lifestyle changes, an aging population, and environmental influences, is fueling the demand for advanced molecular diagnostic solutions, including instruments, kits, and reagents, which are becoming essential for early cancer detection.
- As healthcare providers increasingly adopt these advanced diagnostic tools, the oncology testing segment is projected to witness substantial growth.
- Additionally, the introduction of new products and advancements in cancer genome sequencing technologies, such as innovative assays, are expected to further drive the expansion of the market.
Learn more about the key segments shaping this market
Based on end use, the molecular diagnostics market is segmented into hospitals and clinics, diagnostic laboratories, and other end users. The hospitals and clinics segment accounted for the highest market share of 45.9% in 2024.
- The growing number of hospitals and clinics in both developed and developing countries, driven by factors such as population growth, rising prevalence of chronic and infectious diseases, and advancements in molecular testing technologies, is significantly boosting the demand for advanced molecular diagnostic systems in both newly established and existing healthcare facilities.
- Additionally, the expansion of healthcare infrastructure, particularly in developing regions like the Middle East, Africa, and Asia-Pacific, is fostering the adoption of advanced molecular diagnostic techniques, including PCR-based systems, next-generation sequencing (NGS), and other molecular diagnostic tests, within hospital settings.
- For example, the UK's Department of Health and Social Care allocated approximately USD 17.4 million from its Official Development Assistance (ODA) budget for the period 2022-2025 to enhance healthcare infrastructure and medical workforce development in countries such as Kenya, Ghana, and Nigeria.
- Such investments in healthcare infrastructure are expected to drive the adoption of advanced molecular diagnostic technologies in hospitals and clinics.

Looking for region specific data?
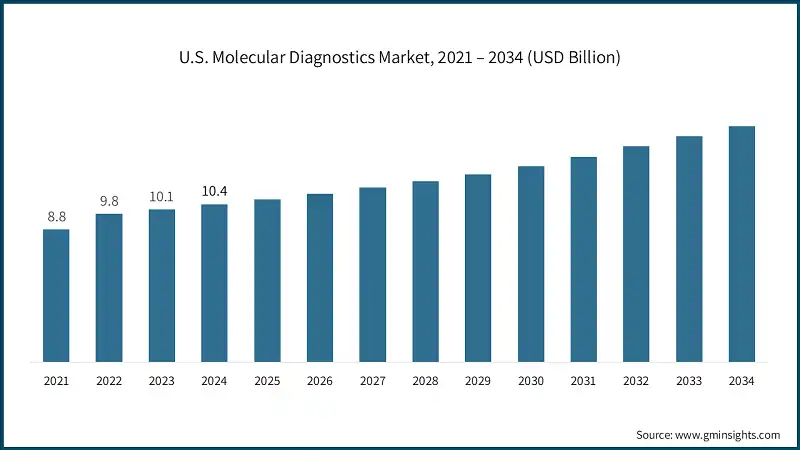
North America dominated the global molecular diagnostics market, with the highest market share of 42.4% in 2024. The region has advanced healthcare infrastructure, and the rate of adoption of innovative molecular diagnostic instruments and technologies is also high. Further, there is an increasing prevalence of infectious disease in the U.S. and Canada, thus propelling the growth of this market.
- The U.S. market was valued at USD 8.8 billion and USD 9.8 billion in 2021 and 2022, respectively. In 2024, the market size reached USD 10.4 billion from USD 10.1 billion in 2023.
- The growth of the market is largely driven by the rising prevalence of infectious diseases in the U.S.
- For example, data from the Centers for Disease Control and Prevention (CDC) indicates that in 2019, the U.S. reported 8,916 cases of tuberculosis, 58,371 cases of salmonella, 34,945 cases of Lyme disease, and 371 cases of meningococcal disease.
- Furthermore, despite a stringent regulatory framework, there is significant support for the approval and commercialization of innovative healthcare solutions, including molecular diagnostic kits and instruments.
- Additionally, advancements in technology and the increasing adoption of advanced molecular diagnostic kits and instruments across the country are further driving market growth.
Europe molecular diagnostics market accounted for USD 6.5 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- One of the key catalysts for the growth of the market in the region is the rising aging population, as the risk of infectious diseases is significantly higher among older adults.
- For instance, according to the European Commission, 21.1% of the EU population was aged 65 or older in 2022, a figure projected to reach 32.5% by 2100. Therefore, as the population ages, the demand for advanced and effective molecular diagnostic kits and instruments in the region is anticipated to rise in the foreseeable future.
Germany molecular diagnostics market is projected to experience steady growth between 2025 and 2034.
- The market in the country is primarily driven by a strong focus on early disease diagnosis, prompting both private and public healthcare professionals to adopt molecular diagnostic tools for precise and efficient disease identification.
- Additionally, the country's well-established healthcare infrastructure and advanced research ecosystem create a conducive environment for the adoption of innovative molecular diagnostic products, further driving market growth.
The Asia Pacific region is projected to be valued at USD 5.6 billion in 2024 and is expected to reach USD 8.8 billion by 2034.
- The market in the region is projected to witness the fastest CAGR, driven by rising awareness of infectious diseases and cancer, expanding government-led screening initiatives, and increasing disposable incomes.
- Additionally, the growing elderly population and improved insurance coverage in the region are further fuelling the demand.
Japan molecular diagnostics market is poised to witness lucrative growth between 2025 – 2034.
- Japan has one of the fastest-aging populations, driving the need for frequent infectious disease screening and monitoring. Molecular diagnostics offer an efficient and reliable solution for infection diagnosis, supporting their increased adoption in the country.
- For instance, projections from the World Economic Forum in 2023 indicated that 1 in 10 individuals in Japan was aged 80 years or older, accounting for nearly one-third of the population.
- This demographic trend has heightened awareness among healthcare providers and policymakers regarding the necessity of effective strategies to address age-related vulnerabilities to infections, thereby contributing to market growth.
Brazil is experiencing significant growth in the molecular diagnostics market.
- The market growth in the country is primarily driven by increasing awareness of infectious disease diagnosis.
- For example, initiatives like the STOP-TB Brazil campaign have significantly enhanced public understanding of tuberculosis (TB), resulting in higher diagnosis rates and reduced stigma associated with the disease.
- As awareness continues to rise, the demand for effective TB molecular diagnostics is expected to grow, thereby contributing to the expansion of the market in the region.
The molecular diagnostics market in Saudi Arabia is expected to experience significant and promising growth from 2025 to 2034.
- Saudi Arabia's well-established healthcare infrastructure, coupled with increasing investments in advanced healthcare technologies, is driving the development and adoption of advanced molecular diagnostic kits and instruments designed to meet the specific needs of the country's patient population.
- Additionally, growing awareness about the significance of early and accurate diagnosis of infectious diseases across various age groups is further fueling market growth in the region.
Molecular Diagnostics Market Share
- Major players such as Abbott Laboratories, F. Hoffmann-La Roche, Danaher Corporation, Siemens Healthineers, and Thermo Fisher Scientific collectively account for 34.6% of the global market share. These companies maintain their market dominance through robust product portfolios, strategic partnerships, regulatory approvals, and continuous innovation. Thermo Fisher Scientific holds a competitive edge due to its extensive product range, widely utilized across various healthcare settings.
- Becton, Dickinson and Company has established a strong position in the market through strategic collaborations and product launches. For example, in June 2022, Becton, Dickinson and Company partnered with CerTest Biotec to develop diagnostic tests for detecting the monkeypox virus. This collaboration enabled the company to expand its product offerings and enhance its research capabilities.
- Additionally, in August 2021, Becton, Dickinson and Company introduced a fully automated, high-throughput diagnostic system in the U.S. This system integrates advanced robotics and sample management software algorithms, setting a new benchmark for automation in molecular testing for infectious diseases in core and centralized laboratories. This innovation has provided BD with a significant competitive advantage in the market.
Molecular Diagnostics Market Companies
Few of the prominent players operating in the molecular diagnostics industry include:
- Abbott Laboratories
- Agilent Technologies
- Becton, Dickinson, and Company
- Biocartis
- Biomerieux
- Bio-Rad Laboratories
- Danaher Corporation
- F. Hoffmann-La Roche
- Hologic
- Huwel Lifesciences
- Illumina
- Qiagen
- QuidelOrtho Corporation
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific
- F. Hoffmann-La Roche
F. Hoffmann-La Roche maintains a strong global presence, operating in over 100 countries and supported by an extensive network of distributors. This robust distribution network significantly enhances the company's market reach and accessibility.
- Abbott Laboratories
Abbott Laboratories maintains a robust product portfolio, driving widespread adoption and contributing to significant market growth. The company offers a range of point-of-care testing products, including the Abbott Panbio COVID-19 Antigen Rapid Test, among others.
- Danaher Corporation
Danaher Corporation employs a robust global workforce of approximately 63,000 individuals, which supports the company's ability to innovate and deliver high-quality solutions effectively.
Molecular Diagnostics Industry News:
- In February 2023, Biocartis, a leading molecular diagnostics company, introduced the Idylla IDH1-2 Mutation Assay Kit (RUO) to select customers. This assay marked the first application of the new Idylla FLEX technology, which separates the generic components of an Idylla test from its test-specific elements. This launch strengthened Biocartis' competitive position in the molecular diagnostics market.
- In May 2022, Abbott secured FDA clearance for its Alinity m STI Assay, a diagnostic test capable of detecting and differentiating four prevalent sexually transmitted infections (STIs): Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV), and Mycoplasma genitalium (MG). The test requires only a single swab or urine sample, which can be collected by a clinician or the patient. Designed to run on Abbott’s Alinity m system, the company’s advanced high-volume molecular diagnostic instrument, the assay utilizes highly sensitive PCR technology for precise infectious disease detection. This product launch enhanced diagnostic efficiency, improved patient outcomes, expanded Abbott’s molecular diagnostics portfolio, and contributed to revenue growth.
The molecular diagnostics market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Product Type
- Instruments
- Reagents and kits
Market, By Technology
- Polymerase chain reaction (PCR)
- Hybridization
- Sequencing
- Isothermal nucleic acid amplification technology (INAAT)
- Microarrays
- Other technologies
Market, By Application
- Infectious disease diagnostics
- COVID-19
- Flu
- Respiratory syncytial virus (RSV)
- Tuberculosis
- CT/NG
- HIV
- Hepatitis C
- Hepatitis B
- Other infectious disease diagnostics
- Genetic disease testing
- Oncology testing
- Other applications
Market, By End Use
- Hospitals and clinics
- Diagnostic Laboratories
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- Saudi Arabia
- South Africa
- UAE
Frequently Asked Question(FAQ) :
What are the upcoming trends in the molecular diagnostics industry?
Key trends include the development of compact, user-friendly molecular diagnostic devices, increasing adoption of point-of-care diagnostics, and advancements in portable RT-PCR systems for field testing and diagnostics.
Which region leads the molecular diagnostics market?
North America dominated the market with a 42.4% share in 2024, driven by advanced healthcare infrastructure and high adoption of innovative molecular diagnostic technologies.
Who are the key players in the molecular diagnostics market?
Key players include Abbott Laboratories, Agilent Technologies, Becton, Dickinson, and Company, Biocartis, Biomerieux, Bio-Rad Laboratories, Danaher Corporation, and F. Hoffmann-La Roche.
Which segment led the molecular diagnostics market?
The infectious disease diagnostics segment led the market with a 71.8% share in 2024.
What was the valuation of the oncology testing segment?
The oncology testing segment was valued at USD 2.1 billion in 2024 and is expected to grow to USD 3.2 billion by 2034.
How much revenue did the polymerase chain reaction (PCR) segment generate in 2024?
The PCR segment generated the highest revenue, accounting for 70.4% of the market share in 2024.
What was the valuation of the hybridization segment in 2024?
The hybridization segment was valued at USD 2.8 billion in 2024 and is projected to grow to USD 4 billion by 2034, with a CAGR of 4%.
What is the market size of the molecular diagnostics in 2024?
The market size was USD 27 billion in 2024, with a CAGR of 4.2% expected through 2034, driven by rising infectious diseases, technological advancements, and increasing demand for point-of-care diagnostics.
What is the projected value of the molecular diagnostics market by 2034?
The market is expected to reach USD 40.4 billion by 2034, fueled by advancements in molecular diagnostic technologies and a growing geriatric population.
What is the projected size of the molecular diagnostics market?
The market size of molecular diagnostics is expected to reach USD 27.9 billion in 2025.
Molecular Diagnostics Market Scope
Related Reports


