Summary
Table of Content

Lysosomal Storage Disease Treatment Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Lysosomal Storage Disease Treatment Market Size
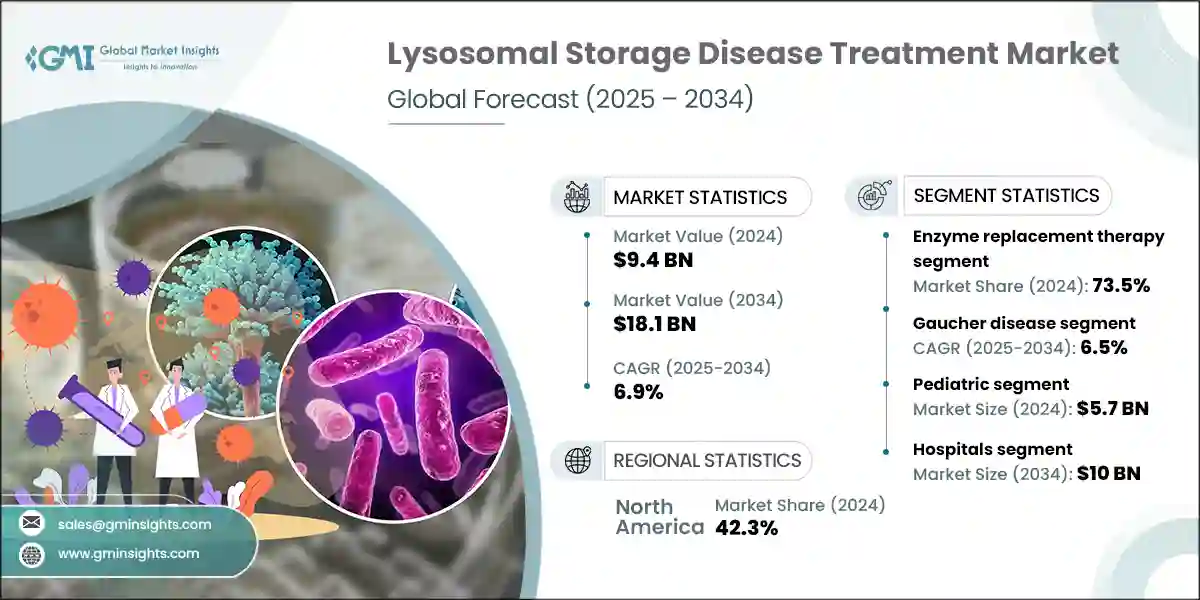
The global lysosomal storage disease treatment market size was valued at USD 9.4 billion in 2024. The market is expected to grow from USD 10 billion in 2025 to USD 18.1 billion in 2034, at a CAGR of 6.9% during the forecast period. This growth is attributed to the increasing incidence of rare genetic disorders such as Gaucher, Fabry, and Pompe disease. In addition, the advancement in enzyme replacement therapy and gene therapy along with regulatory support offering orphan drug designation for lysosomal storage disease drugs, further contributes to market growth.
To get key market trends
The market includes enzyme replacement, gene therapies, and small molecule drugs aimed at improving patient outcomes and quality of life. Major players in the industry are Takeda Pharmaceutical, BioMarin, Orchard Therapeutics, Amicus Therapeutics and Sanofi. These players dominated the market by adopting various strategies such as development of novel treatment modalities, product expansion, and establishing global distribution networks.
Lysosomal Storage Disease Treatment Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 9.4 Billion |
| Forecast Period 2025 - 2034 CAGR | 6.9% |
| Market Size in 2034 | USD 18.1 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing diagnosis due to newborn-screening programs | Increasing diagnosis through newborn-screening programs accelerates market growth by enabling early detection of lysosomal storage diseases, expanding the patient base, and driving demand for timely and effective treatments. |
| Growing pipeline of gene and enzyme therapies | These innovations improve efficacy, address unmet clinical needs, and attract investment, accelerating global adoption and patient access. |
| Advancement in molecular and biomarker-based diagnosis | These technologies improve patient stratification, guide personalized therapies, and enhance monitoring, leading to better outcomes and increased demand for targeted treatments. |
| Increasing shift towards disease-modifying and curative therapies | An increasing shift toward disease-modifying and curative therapies is transforming the lysosomal storage disease treatment market by focusing on long-term solutions rather than symptom management. |
| Pitfalls & Challenges | Impact |
| High cost of treatment | Advanced therapies like enzyme replacement and gene therapy are expensive, limiting access for many patients. This financial burden affects healthcare systems, slows adoption rate of treatment in low- and middle-income regions. |
| Limited penetration in low-and middle-income countries (LMICs) | These challenges restrict early diagnosis and timely intervention, highlighting the need for global health initiatives, affordable treatment options, and improved healthcare delivery systems to bridge the accessibility gap. |
| Opportunities: | Impact |
| Growing demand of gene therapy and genome editing | Growing demand for gene therapy and genome editing offers promising future opportunities in the market by enabling curative, personalized solutions. These innovations drive research investment, expand treatment options, and improve long-term patient outcomes. |
| Rise in specialized rare disease treatment centers | Rise in specialized rare disease treatment centers is boosting the market by improving access to expert care, advanced diagnostics, and tailored therapies. |
| Market Leaders (2024) | |
| Market Leaders |
~22% market share in 2024 |
| Top Players |
Collective Market Share in 2024 is 65% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
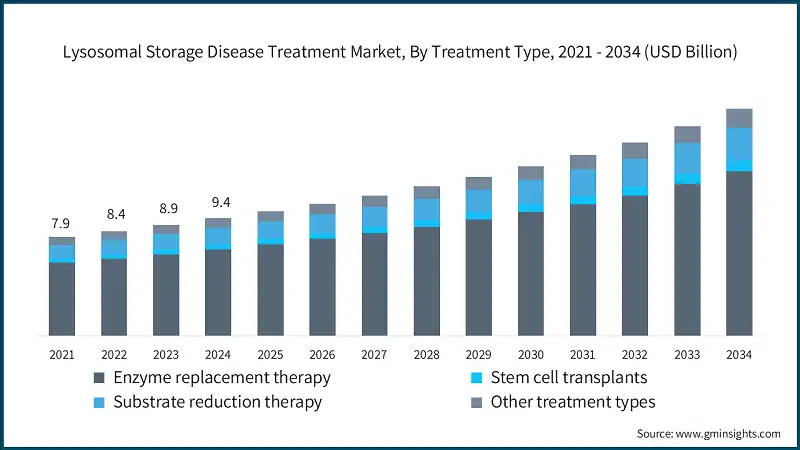
The lysosomal storage disease treatment market witness steady growth, growing from USD 7.9 billion in 2021 to USD 8.9 billion in 2023. The growth of the market is driven by rising incidence of lysosomal storage disease, that further drives the demand for early detection diagnostic tools and effective therapeutics. For instance, as per the report published by the National Institute of Health (NIH), over 50 different lysosomal storage disease (LSDs) have been identified, collectively affecting 1 in 5,000 and 1 in 8,000 births globally. Thus, the growing advancement in diagnostic technologies such as enzyme assays and genetic screening have significantly improved disease identification, leading to higher treatment rates and growing market size.
Further, the increasing number of awareness campaigns, and the expansion of newborn-screening programs have led to earlier detection of LSDs, which is crucial for better clinical outcomes. As an example, the U.S. cities such as New York, and Missouri have included Pompe and Krabbe disease in newborn-screening panels. This shift is significantly increasing the number of patients entering treatment earlier in life, supporting market expansion for both diagnostics and therapeutics, thereby reshaping the future landscape of the market.
Moreover, the emergence of gene therapy and novel treatment modalities such as next-generation treatment platforms are reshaping the LSD treatment landscape. For instance, companies such as Amicus Therapeutics, Avrobio and Sangamo are developing gene therapies for Fabry, Pompe and Gaucher disease.
In September 2023, Amicus Therapeutics announced that the U.S. Food and Drug Administration (FDA) has approved Pombiliti (cipaglucosidase alfa-atga) + Opfolda (miglustat) 65mg capsules. This two-component therapy is indicated for adults living with late-onset Pompe disease (LOPD). Late-onset Pompe disease is a rare, debilitating, and life-threatening lysosomal disorder caused by a deficiency of the enzyme acid alpha-glucosidase (GAA). Therefore, these curative approach promise long-term benefits compared to conventional therapies, thus attracting investment from key stakeholders and boosting market growth.
Lysosomal storage disease (LSD) treatment focuses on managing the symptoms and slowing the progression of these genetic disorders, which are caused by deficiencies in specific enzymes that break down cellular waste products within lysosomes. Treatment options include enzyme replacement therapy, substrate reduction therapy, hematopoietic stem cell transplantation, and gene therapy.
Lysosomal storage diseases (LSDs) cause a toxic buildup that damages the body’s cells and organs. Researchers have found more than 70 types of LSDs. Providers usually diagnose LSDs during pregnancy or infancy.
Lysosomal Storage Disease Treatment Market Trends
- The global market experiences dynamic transformation driven by the rising prevalence of Gaucher, Fabry, Pompe and other genetic and rare disorders, rising global investment in rare disease treatment and increasing shift towards precision and gene therapies.
- Government and pharma companies are significantly increasing funding for rare disease research. Moreover, the growing initiatives such as NIH, Rare Disease Clinical Research Network and EU’s Horizon programs, push innovation in gene therapy and enzyme replacement therapies accelerating market approval and patient access globally.
- Furthermore, the lysosomal storage disease treatment market is experiencing significant growth owing to rising demand for precision and gene therapies. In addition, the ongoing research and development activities to improve treatment options from traditional ERTs towards gene therapies and pharmacological chaperones, drives the growth of the market. Research into lysosomal storage disorders is now helping fuel another biotech boom. Nearly two dozen companies are testing, or plan to test, genetic medicines for lysosomal storage disorders, according to a recent report from Cowen, an investment bank.
- Collectively, gene therapy programs aimed at inherited metabolic diseases mostly lysosomal storage disorders, account for 23% of the roughly 280 gene therapies in the pipeline that Cowen identified. Only treatments for neurological illnesses represented a greater share.
- Therefore, the ongoing clinical trials on gene therapy help to address the root genetic cause, offering potentially curative options, especially for Gaucher and Fabry diseases, reshaping the long-term treatment paradigms.
- Moreover, the global regulatory environment is increasingly favorable with increasing number of approved drugs between 2015 to 2023 receive orphan drug designation from the FDA and EMA. This encourages investment and faster commercialization of novel therapies, particularly for high-burden disorders like Pompe disease and mucopolysaccharidoses.
- Further, the integration of AI and digital health is transforming diagnosis and monitoring in LSDs, digital biomarkers and AI models are being used to identify phenotypic variation and predict progression in diseases like Niemann-Pick and MLD.
- In addition, the increasing collaboration between health organizations, pharma companies and public health bodies to raise awareness about LSDs leads to diagnosis of new cases, that raises the demand for novel treatment options, thereby contributing to market growth.
- Lastly, biopharmaceutical improvements such as continuous processing, single-use bioreactors, and improved cell lines are reducing production costs and improving consistency for biologics like ERTs and gene therapies. These technological advancement support scalability and regulatory compliance, reduces the production cost and make LSDs therapeutics more feasible for a global patient population.
Lysosomal Storage Disease Treatment Market Analysis
Learn more about the key segments shaping this market
In 2021, the global market was valued at USD 7.9 billion. The following year, it saw a slight increase to USD 8.4 billion, and by 2023, the market further climbed to USD 8.9 billion.
Based on treatment type, the global market is divided into enzyme replacement therapy, stem cell transplants, substrate reduction therapy and other treatment types. The enzyme replacement therapy segment accounted for 73.5% of the market in 2024 due to increasing diagnosis rates and early screening programs, and growing prevalence of lysosomal storage disease. The segment is expected to exceed USD 13.1 billion by 2034, growing at a CAGR of 6.7% during the forecast period. On the other hand, the stem cell transplants segment is expected to grow with the fastest CAGR of 7.6%. The growth of this segment can be attributed to curative potential of hematopoietic stem cell transplant, advancement in conditioning regimens and graft techniques and supportive regulatory and funding landscape.
- The growth of newborn-screening and early diagnostic programs has significantly increased the identification of LSDs, therefore raises the demand for enzyme replacement therapies. For instance, the expansion of new born screening programs in U.S. has led to early detection of Gaucher and Pompe disease. According to a report published by the Centre for Disease Control and Prevention, newborn-screening programs detects over 12,000 infants annually with rare conditions, enhances eligibility for timely ERT intervention.
- Moreover, another major factor that drives the growth of the market include the high efficacy demonstrated by ERTs in managing enzyme-deficient lysosomal disorders, leading to improved patient outcomes. In addition, pharmaceutical companies are increasingly investing in enzyme replacement therapies through partnership and in-house research and development. For instance, in June 2025, Chiesi Group and Key2Brain announced a worldwide license agreement to advance the development of two blood-brain barrier (BBB)-crossing recombinant enzyme replacement therapies (ERT) for lysosomal storage disorders (LSD), including alpha-mannosidosis (aMann) and Krabbe disease (KD), ultra-rare diseases that affect the central nervous system.
- The increasing number of partnership and collaboration strengthened the companies ERT pipeline, thereby contributing market growth.
- Additionally, the rising healthcare infrastructure with growing awareness for Fabry, Gaucher and Pompe disease in developing economies such as Brazil, India and China are expanding access to ERT. Government are increasing supporting rare disease programs to raise awareness, that has led to increase in the number of LSDs cases globally, raising the demand for therapeutic interventions such as ERTs therapies.
Based on disease type, the lysosomal storage disease treatment market is classified into Gaucher disease, mucopolysaccharidoses, Pompe disease, Fabry disease, and other disease types. The Gaucher disease segment dominated the market in 2024 and is growing with a CAGR of 6.5% during the forecast period.
- The segment is gaining significant traction due to the high prevalence of Gaucher disease, raises the demand for long-term treatment options such as enzyme replacement therapy and substrate reduction therapy. For instance, as per the National Gaucher Foundation, the global incidence of type 1 Gaucher disease is 1 in 40,000 to 60,000 live births.
- Further, the availability of FDA-approved ERTs such as Imiglucerase, Velaglucerase and Taliglucerase has significantly improved the management of Gaucher. Moreover, these therapies have been shown to reduce spleen and liver size, improve anemia and reduce bone complications.
- The second-largest segment, mucopolysaccharidoses, held a market share of 27.4% in 2024. The market growth for mucopolysaccharidoses is driven by supportive regulatory framework and orphan drug incentives, offering tax credits, enabling accelerated approval pathways, and incentivizing research and development for MPS therapies. In addition, pharma and biotech investment in MPS treatment development including cell and engineered B-cell therapies and gene editing programs are surging, raises the need for expanded laboratory infrastructure for storage, assay development, and clinical trial support.
- Pompe disease segment, though smaller with a market share of 14.5%, is expected to grow with the fastest CAGR of 7.4%, due to rising implementation of newborn-screening for Pompe disease across developed markets such as U.S. and parts of Europe. As an example, as of 2024, all 50 states in U.S. include Pompe disease in their screening panels. Therefore, the early detection of disease enables timely initiation of treatment contributing to market growth.
Based on age group, the lysosomal storage disease treatment market is bifurcated into pediatric and adult. The pediatric segment was anticipated to be worth of USD 5.7 billion in 2024 and is expected to grow at 7.1% CAGR during the forecast period.
- Lysosomal storage disease often occurs during infancy or early childhood, prompting early medical intervention. According to NIH data, over 50% of LSD cases present symptoms within the first year of life, necessitating prompt diagnosis and treatment in the pediatric population.
- This drives the demand for early therapeutic options, particularly enzyme replacement and substrate reduction therapies aimed at reducing disease progression.
- Moreover, pharmaceutical companies are developing pediatric-friendly formulations such as oral dispersible tablets, flavored suspension or miniaturized doses. For example, Sanofi and Takeda introduced pediatric compatible ERTs for Gaucher and Fabry disease treatment.
- This innovation helps improving compliance among pediatric patients, making treatment more accessible, thereby fueling the market growth.
Learn more about the key segments shaping this market
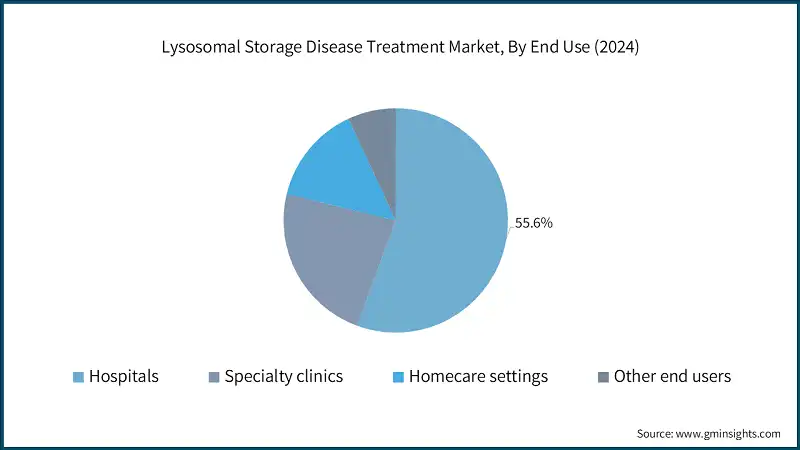
Based on end use, the lysosomal storage disease treatment market is classified into hospitals, specialty clinics, homecare setting and other end users. The hospitals segment dominated the market in 2024 and is expected to reach USD 10 billion by 2034.
- Hospitals are the primary centers for administering complex therapies such as ERTs, which are the mainstay in LSD treatment. These therapies require continuous monitoring, infusion protocols, and emergency care.
- In addition, tertiary hospitals and academic centers are equipped with genetic counseling, diagnostic imaging, and newborn-screening programs, enabling early identification of LSDs.
- Moreover, LSDs often involve multiple organ system requiring treatment from neurology, nephrology, cardiology and metabolic specialists. Also, hospital provide integrated care teams, making them the preferred location for comprehensive treatment, thereby improving patient adherence and compliance.
- Hospitals are primary sites for ongoing clinical trials of next-generation therapies like gene therapy, pharmacological chaperone, and substrate reduction therapies. As an example, as per the ClinicalTrials.gov data, over 85% of active LSD trials are conducted in academic or tertiary hospitals setting, fostering innovation and increasing patient enrollment in advanced treatment options within hospitals, thereby driving market growth.
Looking for region specific data?
The North America lysosomal storage disease treatment market dominated the global market with a market share of 42.3% in 2024.
- The region hosts major players such as Sanofi, Takeda, and Amicus Therapeutics with active research and development and commercial operations in LSDs. Their investment in clinical trials and commercialization pipeline drive treatment accessibility. For instance, Sanofi, a key players in this region, has developed multiple therapies for Gaucher and Fabry disease.
- Moreover, the increasing coverage of high-cost rare disease drugs under public and private insurance plans in U.S. and Canada, drives the growth of the market.
- In addition, institutes like NIH and Canadian Institute of Health Research (CIHR) are actively funded by various public and government organization for LSD-related research. The NIH Rare Disease Clinical Research Network (RDCRN) has allocated millions in grants to LSD studies. These funding supports therapeutic innovation in lysosomal disorders, driving advancement and future pipeline expansion.
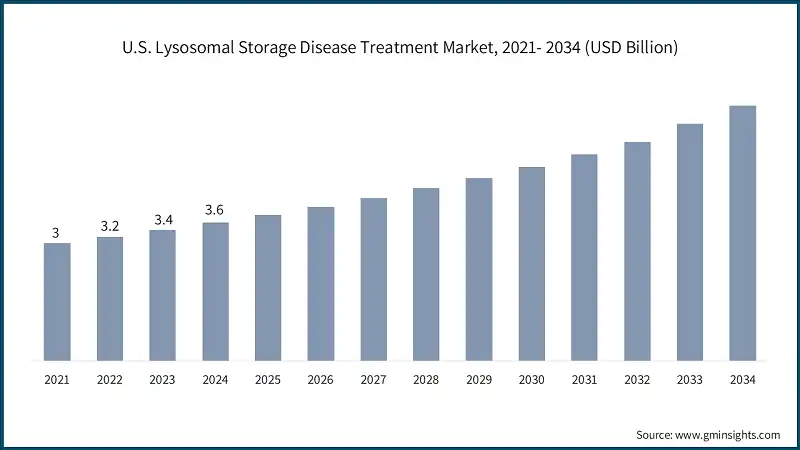
The U.S. market was valued at USD 3 billion and USD 3.2 billion in 2021 and 2022, respectively. The market size reached USD 3.6 billion in 2024, growing from USD 3.4 billion in 2023.
- The U.S. market benefits from robust support from orphan drug development. As of 2024, number of drugs received orphan drug designation in U.S. for LSD-related conditions such as Gaucher, Fabry and Pompe disease. These designation encourage pharmaceutical companies to investment more frequently in LSD therapeutics, accelerating clinical research and product availability.
- Moreover, the country has a growing network of rare disease and LSD specific centers of excellence such as those at Children’s Hospital of Philadelphia and Mount Sinai, which provide advanced diagnostic and therapeutic services, driving market growth.
Europe lysosomal storage disease treatment market accounted for USD 2.5 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- European countries such as Germany, the Netherlands, and Sweden have implemented expanded new born screening panels, inclusive of Pompe disease and Gaucher disease. According to EuroGentest, newborn-screening coverage in Europe reached over 95% in 2023. The early diagnosis increases patient lifespans and improves treatment efficacy, which fuels the demand for ERTs and other treatment modalities across the region.
- Western European countries maintain some of the highest per capita healthcare expenditure globally. In 2023, Germany and France spent over USD 6,300 per capita on healthcare. The increasing spending on healthcare provide access to expensive LSDs treatment, contributing to market growth.
Germany dominates the European market, showcasing strong growth potential.
- Germany has a strong rare disease framework backed by BfArM (Federal Institute for Drugs and Medical Devices). Orphan drugs benefit from fast-track approvals and market exclusivity, encouraging innovation in LSD therapies.
- Moreover, the country’s advanced healthcare infrastructure ensures early diagnosis of LSDs through widespread newborn-screening and genetic testing. In addition, the strong reimbursement system and high healthcare expenditure remove financial barriers, ensuring wider access to LSD therapies and bolstering market growth.
The Asia Pacific lysosomal storage disease treatment market is anticipated to grow at the highest CAGR of 7.5% during the analysis timeframe.
- Several global and regional pharmaceutical companies are expanding their clinical research footprint in the APAC region due to lower costs and large patient base. Countries such as India, and China hosts over 200 active rare disease clinical trials, that helps in bringing investigational LSD therapies more promptly to market, increasing treatment options, thereby fueling market growth.
- Moreover, the rise in technological advancement with availability of enhanced diagnostic capabilities have led to higher reported prevalence, which is stimulating demand for LSD therapeutics thereby contributing to market growth.
- In addition, rapid urbanization and expansion of tertiary care hospitals and genetic counseling center in metro areas have facilitated specialized care for LSD, spurring market growth.
India lysosomal storage disease treatment market is estimated to grow with a significant CAGR, in the Asia Pacific market.
- India has seen improve genetic screening and neonatal diagnostic capabilities, particularly in urban centers. This has led to increased identification of rare disorders like LSDs. Moreover, the growing awareness coupled with increasing availability of diagnostic services at tertiary care centers are accelerating early detection of disease, thereby driving the demand for therapeutic intervention such as ERT and others.
- Major healthcare chains like Apollo Hospitals, Fortis and AIIMS are expanding pediatric genetics departments and rare disease care units. This facilitates better disease management infrastructure and multidisciplinary care for LSD patients.
Latin American market, exhibiting remarkable growth during the analysis period.
- The rising awareness by government and advocacy groups increases the diagnosis rate of rare disease in Latin America. Many countries in Latin America have also expanded the universal healthcare coverage that includes high-treatment cost for LSDs. This has led to an increase in treatment options for LSDs, driving steady market demand.
- Moreover, organizations like GEIRA in Brazil and Fundacion Fabry Argentina plays a vital role in pushing for national funding, early screening and access to therapies. These foundations help maintain public awareness for LSDs and available treatment options, thereby contributing to market growth.
Middle East and Africa lysosomal storage disease treatment market experiencing substantial growth in 2024.
- International biopharmaceutical companies are entering the MEA market to tap into unmet needs. Global players such as Takeda, Sanofi, and BioMarin have increased regional footprint through distribution partnership and patient assistance programs. As an example, Takeda launched its rare disease portfolio in the UAE in 2023, aiming to improve LSD therapy access. Therefore, these commercial expansions are stimulating market penetration and improving treatment availability.
- Moreover, countries such as UAE, Qatar, and Kuwait are enforcing mandatory newborn-screening programs that include rare metabolic disorders like Pompe and MPS. For instance, Qatar’s National Newborn-Screening Program covers over 80 rare disease, improving early detection, that significantly raises the demand for LSDs therapies, thereby spurring market growth.
Lysosomal Storage Disease Treatment Market Share
The market is characterized by diverse players competing in the industry. The top 5 players such as Pfizer, Takeda Pharmaceuticals, Sanofi, BioMarin, and Amicus Therapeutics account for approximately 65% of the market share in the moderately consolidated global market. Leading companies in the market achieve success through a blend of strategic initiatives, robust investment in research and development, targeted solutions that address the unique needs of LSD patients, geographic expansion, innovative business strategies, and strict adherence to regulatory standards.
Companies operating in the market are adopting competitive pricing strategies for biologics and pharmaceuticals to make therapies more accessible and expand their market share. To address unmet clinical needs, key players are introducing innovative drug therapies that offer substantial improvements over traditional treatment approaches.
Additionally, leading firms are implementing multi-faceted strategies such as mergers and acquisitions, strategic partnerships, and increased research and development investments to strengthen their market position and meet the growing global demand for LSD treatments. These proactive measures are driving sustained growth and innovation across the industry.
Alongside established global leaders, emerging companies are playing an increasingly pivotal role in shaping the market especially across rapidly developing regions such as Asia-Pacific and Latin America. These newer entrants may not directly impact the market; however, their innovative treatment approaches and strategic regional expansions are transforming the competitive landscape. This shift is fostering greater market diversity and intensifying competition, ultimately driving progress and accessibility in LSD therapies.
Lysosomal Storage Disease Treatment Market Companies
The company profile section includes both companies that have commercial drug available in the market as well as those that are onto clinical phase development. Prominent players operating in the market are as mentioned below:
- Alexion Pharmaceuticals
- Amicus Therapeutics
- Avrobio
- BioMarin
- JCR Pharmaceuticals
- Johnson & Johnson (Actelion Pharmaceuticals)
- Orchard Therapeutics
- Orphazyme
- Pfizer
- Sanofi (Genzyme Corporation)
- Sigilon Therapeutics
- Sangamo therapeutics
- Takeda Pharmaceutical Company (Shire Plc)
- Pfizer
Pfizer leverages its global research and development infrastructure and regulatory expertise to develop innovative therapies for rare diseases, including LSDs. Its strategic collaborations and focus on biologics enable it to deliver targeted treatments. With a strong pipeline and commitment to accessibility, Pfizer is expanding its footprint in the LSD market through precision medicine and next-gen therapeutic platforms.
- Amicus Therapeutics
Amicus Therapeutics focuses on next-generation treatments for Fabry and Pompe diseases using pharmacological chaperones and precision medicine. Its agile research and development model and strong patient advocacy integration enable rapid development of targeted therapies. Amicus stands out for its commitment to improving quality of life through novel approaches that go beyond conventional LSD treatments.
- Takeda Pharmaceutical
Takeda, through its acquisition of Shire, holds a leading position in LSD treatment with a robust portfolio of enzyme replacement therapies. Its global reach and patient-centric approach drive innovation in Gaucher, Fabry, and Hunter syndromes. Takeda’s continued investment in rare disease research and strategic partnerships reinforces its dominance and commitment to improving long-term patient outcomes.
Lysosomal Storage Disease Treatment Industry News
- In August 2023, An Italian pharmaceutical company and a Boston-area biotechnology research firm announced a partnership to advance a novel blood-brain barrier-crossing platform technology for those with lysosomal storage disorders. Chiesi Global Rare Diseases, team up with Aliada Therapeutics on research and development of multiple enzyme cargoes modified with Aliada's Modular Delivery (MODEL) platform, which harnesses endogenous brain endothelial cell transport mechanisms to move large molecule therapeutics across the blood brain barrier (BBB) more quickly and effectively.
- In May 2023, Sangamo Therapeutics, a genomic medicine company, announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to isaralgagene civaparvovec, or ST-920, a wholly owned gene therapy product candidate for the treatment of Fabry disease.
- In April 2023, Centogene N.V., the essential life science partner for data-driven answers in rare and neurodegenerative diseases, announced it has extended its partnership with Takeda to diagnose patients with lysosomal storage disorders (LSDs). CENTOGENE will continue to provide Takeda with access to diagnostic testing for patients around the world. The aim of the commercial fee-for-service agreement is to enhance patient access to rapid and reliable diagnostics for LSDs, including Fabry disease, Gaucher disease, and Hunter syndrome.
- In June 2021, Codexis, Inc., a leading enzyme engineering company enabling the promise of synthetic biology, announced the expansion of its strategic collaboration and license agreement with Takeda Pharmaceutical Company Limited for the research and development of an additional gene therapy for a lysosomal storage disorder bringing the total number of programs under the agreement to four.
The lysosomal storage disease treatment market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2021 - 2034 for the following segments:
Market, By Treatment Type
- Enzyme replacement therapy
- Stem cell transplants
- Substrate reduction therapy
- Other treatment types
Market, By Disease Type
- Gaucher disease
- Mucopolysaccharidoses
- Pompe disease
- Fabry disease
- Other disease types
Market, By Age Group
- Pediatric
- Adult
Market, By End Use
- Hospitals
- Specialty clinics
- Homecare settings
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which region holds the largest share in the lysosomal storage disease treatment industry?
North America accounted for 42.3% of the market in 2024, with the U.S. contributing USD 3.6 billion, supported by strong R&D activity and insurance coverage for rare diseases.
Who are the key players in the lysosomal storage disease treatment market?
Leading players include Takeda Pharmaceuticals, Pfizer, Sanofi, BioMarin, and Amicus Therapeutics. Other notable companies are Alexion, Avrobio, Sangamo, and Orchard Therapeutics.
Which end-use segment contributed the most to lysosomal storage disease treatment market revenue in 2024?
Hospitals led the market in 2024 and are projected to reach USD 10 billion by 2034, due to their role in complex ERT administration and clinical trial activity.
Which age group led the market in 2024?
The pediatric segment dominated in 2024 with USD 5.7 billion in revenue, driven by early onset of LSDs and the development of child-friendly treatment formulations.
What is the fastest growing disease segment during the forecast period?
The Pompe disease segment is expected to grow at the highest CAGR of 7.4% due to expanded newborn-screening programs and early treatment initiatives.
What is the growth outlook for stem cell transplant treatments through 2034?
Stem cell transplant treatments are projected to grow at the fastest CAGR of 7.6% through 2034, owing to their curative potential and regulatory support.
Which disease type dominated the lysosomal storage disease treatment market in 2024?
Gaucher disease held the largest share in 2024, driven by the availability of FDA-approved ERTs and increasing global diagnosis rates.
How much revenue did the enzyme replacement therapy segment generate in 2024?
Enzyme replacement therapy generated USD 6.9 billion in 2024, accounting for 73.5% of the market.
What is the market size of the lysosomal storage disease treatment in 2024?
The market size was USD 9.4 billion in 2024, driven by rising diagnosis rates, early screening programs, and increasing prevalence of Gaucher, Fabry, and Pompe diseases.
What is the projected value of the lysosomal storage disease treatment market by 2034?
The market is expected to reach USD 18.1 billion by 2034, growing at a CAGR of 6.9%, fueled by gene therapy innovations and orphan drug designations.
Lysosomal Storage Disease Treatment Market Scope
Related Reports


