Summary
Table of Content

Immunotherapy Drugs Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Immunotherapy Drugs Market Size
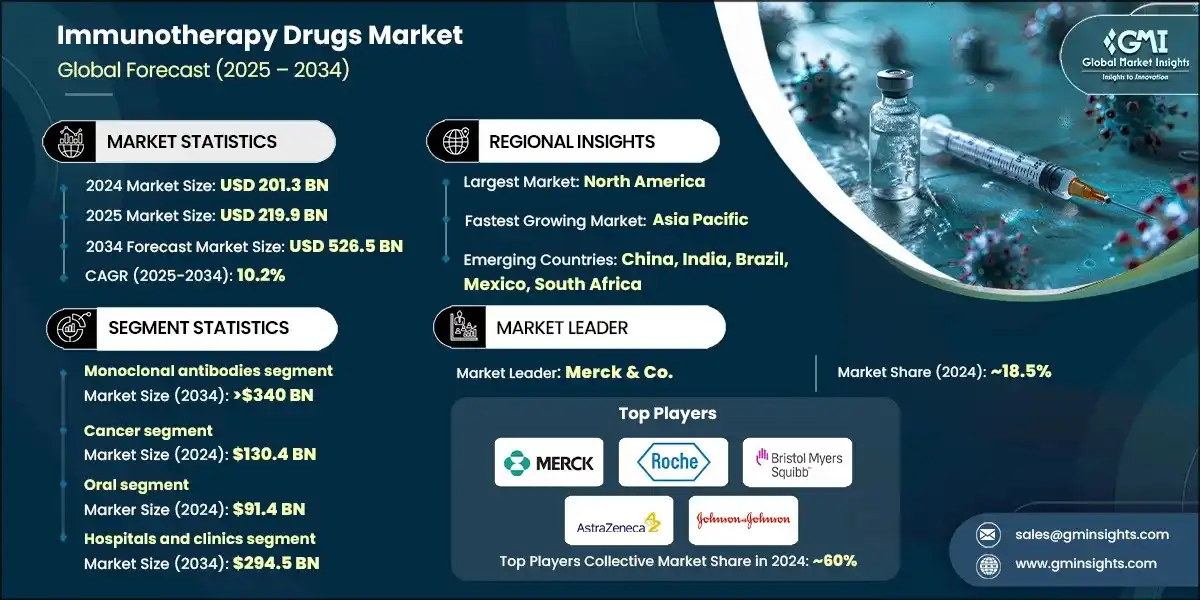
The global immunotherapy drugs market size was valued at USD 201.3 billion in 2024. The market is expected to grow from USD 219.9 billion in 2025 to USD 526.5 billion in 2034, at a CAGR of 10.2% during the forecast period, according to the latest report published by Global Market Insights Inc.
To get key market trends
This growth is attributed to the rising prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases, along with advancement in antibody engineering. Technologies like checkpoint inhibitors, CAR-T cell therapy, and bispecific antibodies are revolutionizing cancer treatment protocols. Moreover, AI-driven drug discovery and biomarker-based patient stratification are accelerating clinical development and improving outcomes.
Additionally, patients and healthcare providers are increasingly favoring personalized medicine, which tailors treatment based on individual genetic and molecular profiles. In addition, precision therapies, such as neoantigen vaccines and biomarker-guided regimens, are gaining traction in oncology and beyond. This shift is supported by advancements in genomics, proteomics, and diagnostic tools that enable better patient selection and monitoring, that further contribute to market growth.
Immunotherapy drugs are a type of treatment that uses substances to stimulate or suppress the immune system to help the body fight diseases like cancer, infections, and autoimmune disorders. They work by either boosting the body's natural immune response or by adding lab-made substances, such as proteins or antibodies, that act as part of the immune system. Major players in the industry are F. Hoffmann La Roche, Merck & Co., Bristol Myers Squibb, AstraZeneca and Johnson & Johnson. These players dominated the market by adopting various strategies such as product expansion and establishing global distribution networks.
Immunotherapy drugs market has witnessed steady growth, growing from USD 152 billion in 2021 to USD 183 billion in 2023. Global regulatory bodies such as the U.S. FDA and EMA are increasingly offering fast-track, breakthrough therapy, and orphan drug designations to immunotherapy products. These pathways accelerate the development and approval of novel treatments, especially for life-threatening conditions like cancer and rare autoimmune diseases, fueling the growth of the market.
Additionally, the immunotherapy sector is witnessing a surge in strategic partnerships, mergers, and acquisitions among biotech firms, pharmaceutical giants, and academic institutions. These collaborations help pool resources, share risks, and accelerate the development of next-generation immunotherapies like CAR-T cells and bispecific antibodies. For instance, the National Cancer Institute reported that collaborative research partnerships in immunotherapy increased by 32% between 2021 and 2024. These collaborations enable resource sharing, risk distribution, and faster development of advanced immunotherapies, including CAR-T cells and bispecific antibodies.
While oncology remains the primary focus, immunotherapy is rapidly expanding into other therapeutic areas such as autoimmune diseases, infectious diseases, and allergies. Drugs like monoclonal antibodies and cytokine modulators are being repurposed or newly developed for conditions like rheumatoid arthritis, multiple sclerosis, and even COVID-19.
Immunotherapy Drugs Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 201.3 Billion |
| Market Size in 2025 | USD 219.9 Billion |
| Forecast Period 2025 – 2034 CAGR | 10.2% |
| Market Size in 2034 | USD 526.5 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising prevalence of chronic diseases | The global surge in chronic illnesses, especially cancer, autoimmune disorders, and infectious diseases, is a major driver of market growth. |
| Technological advancement in antibody engineering | Technologies like checkpoint inhibitors, CAR-T cell therapies, and bispecific antibodies are revolutionizing cancer treatment protocols. |
| Growing demand for personalized and targeted therapies | Precision therapies, such as neoantigen vaccines and biomarker-guided regimens, are gaining traction in oncology and beyond. |
| Expansion into non-oncology applications | Drugs like monoclonal antibodies and cytokine modulators are being repurposed or newly developed for conditions like rheumatoid arthritis, multiple sclerosis, and even COVID-19. |
| Pitfalls & Challenges | Impact |
| High cost and limited accessibility | The lack of cost-effective production methods and scalable infrastructure remains a significant barrier to widespread use, making affordability a critical issue for long-term market sustainability. |
| Variable patient response and resistance | Without precise diagnostics, clinicians may struggle to match patients with the most effective therapies, leading to suboptimal results and increased healthcare costs. |
| Opportunities: | Impact |
| Decentralized manufacturing and regional hubs | To overcome logistical and cost barriers, companies are establishing decentralized manufacturing models, especially in Asia-Pacific and Canada. |
| Personalized neoantigen vaccines | These vaccines are gaining traction in clinical trials for melanoma, lung, and bladder cancers. As sequencing costs drop and bioinformatics improve, this niche is expected to become a major growth area. |
| Market Leaders (2024) | |
| Market Leaders |
~18.5% market share. |
| Top Players |
Collective market share in 2024 is ~60% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Immunotherapy Drugs Market Trends
- The global rise in cancer incidence, with over 20 million new cases expected annually by 2030, is a major macroeconomic driver of immunotherapy. Aging populations, particularly in developed nations, are more susceptible to cancer and autoimmune diseases, increasing demand for advanced therapies.
- In addition, the immunotherapy landscape is increasingly shaped by biomarker-driven strategies that enable personalized treatment. Biomarkers like PD-L1 expression, tumor mutational burden (TMB), and microsatellite instability (MSI) are now routinely used to guide therapy selection. For instance, pembrolizumab’s approval for MSI-high tumors regardless of origin marked a paradigm shift. For instance, the UK’s NHS is piloting mRNA cancer vaccines tailored to individual tumor profiles. This trend enhances treatment efficacy and reduces adverse effects, aligning with value-based care, that further contributes to market growth.
- Next-generation immunotherapies are leveraging multi-specific antibodies and gene-edited immune cells to overcome tumor resistance and heterogeneity. For example, tri-specific antibodies can simultaneously target multiple tumor antigens and immune checkpoints, enhancing efficacy. CAR-T cell therapy has evolved into fifth-generation constructs with JAK/STAT signaling and cytokine secretion capabilities.
- Additionally, CAR-NK and CAR-Macrophage therapies are being developed to improve tumor infiltration and reduce toxicity. These innovations are reshaping the therapeutic landscape, with over 50 multi-specific antibody trials currently underway globally.
- Regulatory bodies such as the U.S. FDA are accelerating immunotherapy approvals through fast track and breakthrough therapy designations. In 2025, EO2463, an AI-designed immunotherapy for follicular lymphoma, received Fast Track status after showing a 46% objective response rate in early trials.
- Similarly, pamlectabart tismanitin, an ADC for relapsed multiple myeloma, was fast-tracked due to its novel mechanism and promising efficacy. These pathways allow rolling submissions, real-time data sharing, and priority reviews, reducing development timelines by up to 30%.
- This regulatory agility is crucial for bringing life-saving therapies to market faster, especially for cancers with high unmet needs.
- Manufacturing is undergoing a transformation with decentralized and automated models to meet the growing demand for autologous therapies. Traditional CAR-T production takes 2-3 weeks, but Thermo Fisher’s 24-hour automated workflow using lentiviral vectors and closed systems significantly reduces turnaround time.
- Lastly, decentralized manufacturing at point-of-care centers improves access, especially in remote regions, and reduces logistical burdens. This model is supported by modular, digitally integrated platforms like Gibco’s CTS Cellmation. As CAR-T trials increase to 91 new trials in 2023 alone scalable, localized manufacturing is becoming essential to ensure timely delivery and cost efficiency.
Immunotherapy Drugs Market Analysis
Learn more about the key segments shaping this market
In 2021, the global market was valued at USD 152 billion. The following year, it saw an increase to USD 166.7 billion, and by 2023, the market further climbed to USD 183 billion.
Based on drug type, the global market is divided into monoclonal antibodies, vaccines, interferons alpha & beta, interleukins and other drug types. The monoclonal antibodies segment accounted for 63.3% of the market in 2024. The segment is expected to exceed USD 340 billion by 2034, growing at a CAGR of 10.4% during the forecast period.
- The increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases is fuelling demand for targeted therapies. Monoclonal antibodies offer high specificity and fewer side effects compared to traditional treatments. For example, trastuzumab has significantly improved outcomes in HER2-positive breast cancer. As global cancer cases are projected to exceed 20 million annually by 2030, mAbs are becoming essential in modern therapeutic strategies.
- Technological innovations such as humanized, fully human, bispecific, and antibody-drug conjugates (ADCs) have enhanced the efficacy and safety of mAbs. These engineered antibodies improve immune targeting and reduce off-target effects. For instance, bispecific antibodies such as blinatumomab engage both cancer and immune cells, boosting response rates in leukemia.
- Further, monoclonal antibodies are no longer limited to oncology. They are now widely used in autoimmune diseases (e.g., adalimumab for arthritis), infectious diseases (e.g., COVID-19), and even neurological conditions. Their ability to precisely target disease-specific antigens makes them versatile tools across multiple indications.
- The vaccines segment of the market is projected to grow significantly with a CAGR of 10.2% due to increasing innovation such as peptide-based, cell-based, and nucleic acid-based vaccines are enhancing efficacy and safety. These technologies allow for faster development and personalized immunization strategies. For example, BioNTech and Moderna are advancing mRNA cancer vaccines, with clinical trials underway. Such breakthroughs are expanding the scope of immunotherapy beyond traditional boundaries.
Based on the application, the immunotherapy drugs market is segmented into cancer, autoimmune diseases, infectious disease, and other applications. The cancer segment dominated the market in 2024 with a revenue of USD 130.4 billion.
- The increasing prevalence of cancer is a major growth drive impacting market. According to WHO, over 20 million new cancer cases were reported globally in 2022, projected to reach 24.58 million by 2030. This surge in disease burden has intensified demand for effective treatments. Immunotherapy offers targeted, less toxic alternatives to chemotherapy, making it a preferred option for oncologists and patients alike.
- Technological innovations such as immune checkpoint inhibitors, CAR-T cell therapies, and monoclonal antibodies have revolutionized cancer treatment. For instance, AstraZeneca’s Imfinzi became the first approved regimen for limited-stage small cell lung cancer in 2024. These therapies enhance immune response and improve survival rates, driving their adoption across multiple cancer types.
- In addition, the rise of precision oncology is boosting demand for immunotherapies tailored to individual genetic profiles. Biomarker-driven treatments, such as MEKTOVI and BRAFTOVI for BRAF V600E-mutated NSCLC, exemplify this trend. Personalized immunotherapies improve outcomes and reduce side effects, making them increasingly popular among clinicians and patients.
- The autoimmune diseases segment of the immunotherapy drugs market is projected to grow rapidly due to increasing shift from broad immunosuppression to targeted biologics has revolutionized autoimmune treatment. Therapies like TNF-alpha and IL-17 inhibitors have improved outcomes in diseases like psoriasis and RA. The rise of bispecific antibodies, which target multiple pathways simultaneously, is expanding options for complex conditions.
- Patent expirations of blockbuster biologics like Humira are paving the way for biosimilars, which offer cost-effective alternatives. Biosimilars now account for 25% of new launches in autoimmune therapeutics. This trend is improving access in emerging markets and reducing treatment costs, especially where affordability is a barrier, boosting market growth.
Based on route of administration, the immunotherapy drugs market is classified into intravenous, subcutaneous and oral. The oral segment dominated the market in 2024 with USD 91.4 billion and is growing with a CAGR of 10.6% during the forecast period.
- Oral immunotherapy drugs offer ease of use, eliminating the need for hospital visits or infusion centers. This improves patient compliance, especially for chronic conditions requiring long-term treatment. The flexibility of at-home administration makes oral therapies more appealing, particularly in regions with limited healthcare infrastructure.
- Oral immunotherapy drugs reduce the need for specialized infusion infrastructure, lowering overall treatment costs. They also simplify logistics and distribution, making them more scalable for large patient populations. This is particularly beneficial in emerging markets where healthcare resources are limited. The cost-efficiency of oral therapies supports broader access and adoption, contributing to their growing market share.
- The subcutaneous segment grew with a CAGR of 10.1% during the analysis period. Subcutaneous delivery enables patients to self-administer immunotherapy drugs at home, reducing hospital visits and improving convenience. This is especially beneficial for chronic conditions like rheumatoid arthritis and ulcerative colitis.
- The aging global population is driving demand for less invasive, home-friendly treatment options. Subcutaneous delivery is ideal for elderly patients managing chronic conditions like cancer, diabetes, and autoimmune diseases. According to WHO, the population aged 80+ is expected to triple by 2050, amplifying the need for accessible and manageable therapies like SC immunotherapy.
Learn more about the key segments shaping this market
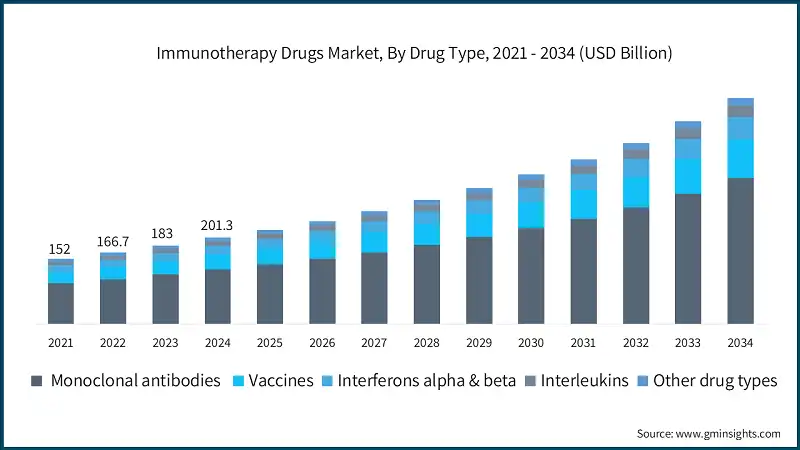
Based on end use, immunotherapy drugs market is classified into hospitals and clinics, cancer research institutes, pharmaceutical and biotechnology companies and other end users. The hospitals and clinics segment dominated the market in 2024 and is expected to reach USD 294.5 billion by 2034.
- Hospitals and clinics are equipped with the necessary infrastructure to administer complex immunotherapies such as CAR-T cells and monoclonal antibodies. These treatments often require infusion centers, monitoring equipment, and trained personnel.
- Hospitals and clinics benefit from structured reimbursement systems that support high-cost immunotherapies. In regions like North America, favorable insurance policies and government programs make it easier for patients to access treatments in hospital settings. This financial support encourages the use of immunotherapy drugs in institutional environments, boosting market share for this segment.
- Hospitals and clinics are key sites for clinical trials and early access programs for new immunotherapy drugs. Their involvement in research accelerates drug adoption and builds trust among healthcare providers. For example, many recent approvals like AstraZeneca’s Imfinzi and Roche’s Tecentriq Hybreza were launched through hospital-based trials, reinforcing their role in driving innovation and uptake.
Looking for region specific data?
North America Immunotherapy Drugs Market
The North America market dominated the global market with a market share of 46.2% in 2024.
- North America faces a significant burden of chronic illnesses, especially cancer, autoimmune disorders, and allergies. In the U.S. alone, over 1.9 million new cancer cases were expected in 2023. Immunotherapy offers targeted, less toxic alternatives to traditional treatments, making it a preferred option. This rising disease incidence is a major driver of market growth in the region.
- North America has a large population affected by allergies, including food allergies. In the U.S., over 32 million people suffer from food allergies, with 200,000 emergency visits annually, yet no FDA-approved immunotherapy exists for this condition. This unmet need presents a major opportunity for therapeutic innovation and market expansion in allergy-focused immunotherapy.
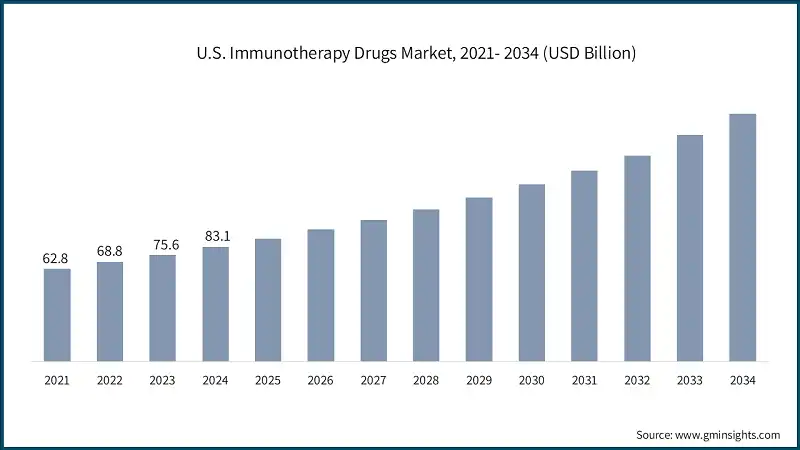
The U.S. immunotherapy drugs market was valued at USD 62.8 billion and USD 68.8 billion in 2021 and 2022, respectively. The market size reached USD 83.1 billion in 2024, growing from USD 75.6 billion in 2023.
- The U.S. leads in immunotherapy innovation, supported by robust research and development investments and biotech collaborations. Companies such as Rise Therapeutics and major players such as Pfizer and Johnson & Johnson are advancing novel therapies. The FDA’s acceptance of IND applications for new immunotherapies highlights the country’s commitment to clinical innovation.
- Combination therapies immunotherapy paired with chemotherapy, radiation, or targeted drugs are gaining traction in the U.S. due to improved patient outcomes. This approach is especially effective in treating resistant cancers like lung and bladder cancer. The FDA has approved several combination regimens, reflected clinical success and expanded the scope of immunotherapy applications.
Europe Immunotherapy Drugs Market Europe market accounted for USD 54.9 billion in 2024 and is anticipated to show lucrative growth over the forecast period. Germany dominates the European immunotherapy drugs market, showcasing strong growth potential. The Asia Pacific market is anticipated to grow at the highest CAGR of 10.5% during the analysis timeframe. China immunotherapy drugs market is estimated to grow with a significant CAGR, in the Asia Pacific market. Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period. Saudi Arabia market to experience substantial growth in the Middle East and Africa market in 2024. The market features a dynamic and moderately consolidated competitive landscape, led by pharmaceutical giants such as F. Hoffmann-La Roche, Merck & Co., Bristol Myers Squibb, AstraZeneca, and Johnson & Johnson, who collectively command approximately 60% of the global market share. Their leadership is driven by strategic investments in research and development, global expansion, and the development of patient-centric therapies targeting cancer, autoimmune disorders, and infectious diseases. To reinforce their market positions, these companies are deploying multi-pronged strategies including mergers and acquisitions, strategic partnerships, and value-based pricing models. These approaches aim to improve access to advanced immunotherapies, enhance affordability, and address unmet clinical needs across diverse geographies. Meanwhile, emerging biotech firms are contributing to market evolution through innovations in drug delivery systems, such as subcutaneous formulations, bispecific antibodies, and AI-driven drug design. Their impact is especially notable in regions like Asia-Pacific, Latin America, and the Middle East, where expanding healthcare infrastructure and rising awareness of immunotherapy are accelerating adoption. As competition intensifies and treatment modalities diversify, companies are evolving their product portfolios to meet global demand for effective, scalable, and personalized immunotherapy solutions, fueling sustained growth and innovation across the market. Prominent players operating in the immunotherapy drugs industry are as mentioned below: Merck leads the market with a share of ~18.5% in 2024. Merck maintains a strong competitive edge through its blockbuster immunotherapy drug Keytruda, which dominates the checkpoint inhibitor space. The company leverages extensive clinical trial networks and combination therapy strategies to expand indications across multiple cancer types. Roche’s competitive strength lies in its diversified immunotherapy portfolio, including Tecentriq and multiple monoclonal antibodies. The company emphasizes real-world evidence, adaptive trial models, and personalized medicine to enhance treatment outcomes. Roche’s CSA includes strategic investments in biomarker platforms, multiplex diagnostics, and combination regimens, enabling it to maintain leadership in both oncology and autoimmune segments while expanding access across Europe and emerging markets. BMS leverages its flagship immunotherapy Opdivo and anticoagulant Eliquis to fund aggressive expansion into neuroscience and radiopharmaceuticals. BMS is focused on executing new product launches, managing patent cliffs, and integrating AI to reduce development timelines, positioning itself for long-term resilience and innovation in immuno-oncology.Asia Pacific Immunotherapy Drugs Market
Latin America Immunotherapy Drugs Market
Middle East and Africa Immunotherapy Drugs Market
Immunotherapy Drugs Market Share
Immunotherapy Drugs Market Companies
Immunotherapy Drugs Industry News
The immunotherapy drugs market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Billion from 2021 - 2034 for the following segments:
Market, By Drug Type
- Monoclonal antibodies
- Vaccines
- Interferons alpha & beta
- Interleukins
- Other drug types
Market, By Application
- Cancer
- Autoimmune diseases
- Infectious diseases
- Other applications
Market, By Route of Administration
- Intravenous
- Subcutaneous
- Oral
Market, By End Use
- Hospitals and clinics
- Cancer research institutes
- Pharmaceutical and biotechnology companies
- Other end users
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which region leads the global immunotherapy drugs industry?
North America led the market with a 46.2% share (USD 92.9 billion) in 2024, driven by strong R&D investment, high disease burden, and supportive reimbursement policies for innovative immunotherapies.
Who are the key players in the global immunotherapy drugs market?
Key players include Merck & Co., F. Hoffmann-La Roche, Bristol Myers Squibb, AstraZeneca, Johnson & Johnson, Amgen, Pfizer, Novartis, Sanofi, Gilead Sciences, GlaxoSmithKline, Moderna, Bluebird Bio, Kite Pharma, and Adaptimmune Therapeutics.
Which end-use segment dominated the global immunotherapy drugs market in 2024?
The hospitals and clinics segment led the market and is expected to reach USD 294.5 billion by 2034, supported by advanced treatment infrastructure.
Which route of administration dominated the global immunotherapy drugs industry in 2024?
The oral segment accounted for the largest share with USD 91.4 billion revenue in 2024, reflecting patient preference for non-invasive treatments, enhanced compliance, and growing development of oral biologics and small-molecule immunomodulators.
Which application segment generated the highest revenue in 2024?
The cancer segment led the market with USD 130.4 billion revenue in 2024, driven by increasing cancer prevalence and the widespread adoption of checkpoint inhibitors, CAR-T therapies, and bispecific antibodies.
What is the market size of the global immunotherapy drugs market in 2024?
The market size was USD 201.3 billion in 2024, driven by the rising prevalence of cancer, autoimmune.
What is the projected market value of the global market by 2034?
The market is expected to reach USD 526.5 billion by 2034, growing at a CAGR of 10.2%, fueled by the expansion of personalized medicine.
What is the estimated market size of the global market in 2025?
The market is projected to reach USD 219.9 billion in 2025, supported by increased clinical adoption of precision immunotherapies.
Which drug type segment dominated the global immunotherapy drugs market in 2024?
The monoclonal antibodies segment held the largest share of 63.3% (USD 127.4 billion) in 2024, driven by their high specificity, expanding applications beyond oncology, and growing use in autoimmune and infectious diseases.
Immunotherapy Drugs Market Scope
Related Reports


