Summary
Table of Content

Home Sleep Apnea Testing Devices Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Home Sleep Apnea Testing Devices Market Size
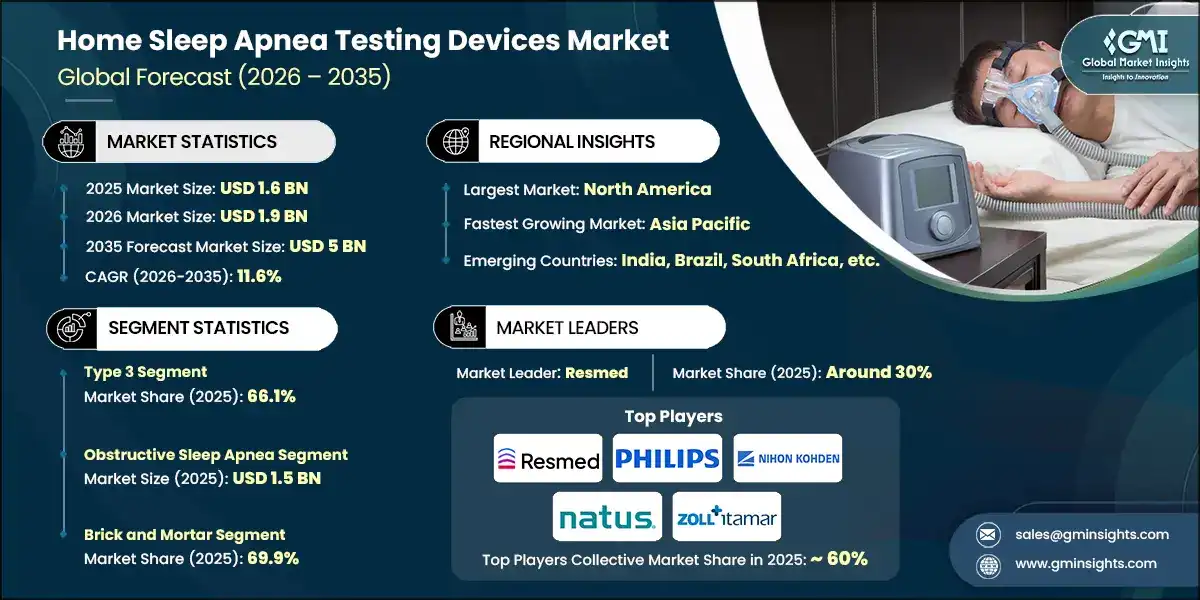
The global home sleep apnea testing devices market size was estimated at USD 1.6 billion in 2025. The market is expected to grow from USD 1.9 billion in 2026 to USD 5 billion in 2035, at a CAGR of 11.6% during the forecast period, according to the latest report published by Global Market Insights Inc.
To get key market trends
The market is driven by the rise in the aging population, growing prevalence of sleep apnea and related co-morbidities, technological advancements in wearable and portable HSAT devices, and increasing awareness regarding sleep health, among other factors.
Convenience and cost-effectiveness of home-based testing vs. in-lab studies and growing adoption of telemedicine and remote patient monitoring are among the key factors propelling the industry demand. Resmed, PHILIPS, NIHON KOHDEN, natus, and ZOLL itamar are among the leading players operating in the market. These players mainly focus on product innovation, integration of advanced technologies, geographic expansion, and collaboration with local or regional healthcare providers, among others.
The market has increased from USD 1.2 billion in 2023 and reached USD 1.6 billion in 2025, with the historic growth rate of 17.9%. Millions of people suffer from sleep apnea around the world. For instance, according to the recently published data, around 1 billion people have sleep apnea globally. The report also highlights that obstructive sleep apnea (OSA) dominates this data, whereas central sleep apnea (CSA) patients are in a significantly smaller ratio.
As obesity, diabetes, and heart disease are all strong indicators for developing sleep apnea, the consistently growing prevalence of these conditions is expected to result in increased demand for early detection and treatment of sleep apnea. Untreated sleep apnea may increase the risk of high blood pressure, stroke, and heart failure, and hence, it is essential to diagnose patients in a timely manner to avoid these complications. HSAT (home sleep apnea testing) is an easier and more affordable method than conducting laboratory tests for patients, which is helping propel the growth of HSAT testing devices.
Furthermore, the continued evolution of sensor technology, wireless connectivity, and artificial intelligence (AI) has made it possible for HSAT devices to be more compact, more accurate, and easier for users to utilize. When used with smartphones and cloud-based applications, modern HSAT devices allow physicians to remotely monitor patients and track results in real-time. Features such as multi-night testing, auto-scores, and predictive algorithms have increased the accuracy of diagnostic testing. It helped in reducing the amount of manual work done by clinicians, resulting in improved patient compliance and comfort, thereby increasing the attractiveness of HSAT devices compared to polysomnography. Continued research and development into miniaturization and the rise of intelligent wearable technology are anticipated to fuel the adoption of HSAT devices over the analysis period.
Home Sleep Apnea Testing (HSAT) devices are portable diagnostic tools designed to monitor breathing patterns, airflow, oxygen saturation, and related parameters at home, enabling convenient detection of sleep apnea without in-lab polysomnography.
Home Sleep Apnea Testing Devices Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2025 |
| Market Size in 2025 | USD 1.6 Billion |
| Market Size in 2026 | USD 1.9 Billion |
| Forecast Period 2026 - 2035 CAGR | 11.6% |
| Market Size in 2035 | USD 5 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Growing prevalence of sleep apnea and related co-morbidities | The increasing number of patients with sleep apnea and associated conditions like obesity and cardiovascular diseases is creating strong demand for accessible diagnostic solutions, significantly boosting HSAT adoption globally. |
| Increasing awareness regarding sleep health and home sleep apnea tests | Public education campaigns and telehealth platforms have improved awareness, leading to higher patient engagement and preference for home-based testing, accelerating market penetration of HSAT devices. |
| Rising aging population | Older adults face higher risk of sleep disorders, and mobility challenges make home testing more appealing. This demographic shift is driving demand in developed and emerging markets. |
| Technological advancements in wearable and portable HSAT devices | Innovations in miniaturization, wireless connectivity, and AI-driven analytics have enhanced device accuracy and usability, making HSAT more attractive to patients and providers. |
| Pitfalls & Challenges | Impact |
| Stringent regulatory approvals for diagnostic devices | Complex compliance requirements delay product launches and increase development costs, slowing market entry for new players and limiting innovation speed. |
| Limited accuracy compared to in-lab polysomnography | HSAT devices cannot fully replicate comprehensive sleep studies, reducing physician confidence for complex cases and restricting adoption in certain patient segments. |
| Opportunities: | Impact |
| Development of AI-powered diagnostic algorithms | AI integration will enable automated scoring, predictive analytics, and personalized treatment recommendations, improve diagnostic accuracy and expand HSAT use in diverse patient populations. |
| Growth of telehealth platforms offering HSAT services | Telemedicine expansion will make HSAT devices more accessible, streamline data sharing with clinicians, and support remote care models, driving widespread adoption and market scalability. |
| Market Leaders (2025) | |
| Market Leaders |
Around 30% |
| Top Players |
|
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing Market | Asia Pacific |
| Emerging countries | India, Brazil, South Africa, etc. |
| Future outlook |
|
What are the growth opportunities in this market?
Home Sleep Apnea Testing Devices Market Trends
Integration of AI and predictive analytics, miniaturization and wearable technology, subscription-based service models, telehealth and remote monitoring adoption, and cloud-based data management are among the key trends shaping the market growth in an upward trajectory.
- Algorithms using artificial intelligence have improved diagnosis accuracy, due to their ability to automate scoring and recognize complex sleep data patterns. Predictive analytics allow for customized treatment plans, reducing the need for manual interpretation and enhancing clinical efficiency.
- Additionally, the evolution and advancement of sensor technology has resulted in smaller, more comfortable HSAT (home sleep apnea test) devices that easily connect to smartphones. Patients may feel encouraged to use these devices because they provide a more complete picture of their sleep and allow for multi-night testing, which results in a significantly more accurate diagnosis.
- Telemedicine has increased the usage of Home Sleep Apnea Tests (HSAT), as it has enabled remote consultations and sharing of test results. Patients can perform home testing of their sleep apnea condition, while physicians evaluate test results online, thus increasing patient access to physicians and eliminating the need for patients to visit the hospital for their HSAT.
- Furthermore, as HSAT device manufacturers offer subscription-based package deals that include hardware, software, and data analytics, consumers need to pay less upfront for their devices, and manufacturers may have a recurring revenue stream from these subscriptions. This method is anticipated to improve the affordability of HSAT devices to patients.
Home Sleep Apnea Testing Devices Market Analysis
Learn more about the key segments shaping this market
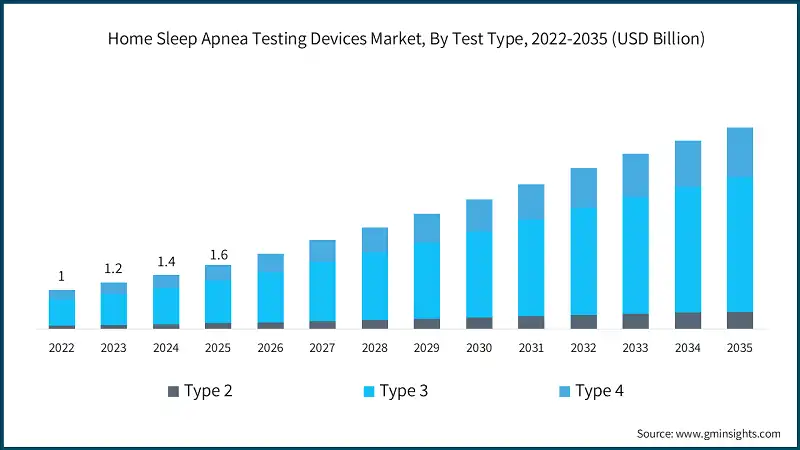
In 2022, the market was valued at USD 1 billion and grew to USD 1.2 billion in 2023, reaching USD 1.4 billion by 2024. Increasing healthcare expenditure and focus on preventive care coupled with multi-night testing capabilities for better accuracy are among the key variables contributing to industry growth.
Based on test type, the global market is segmented into type 2, type 3, and type 4. The type 3 segment accounted for a leading share of 66.1% in 2025. Increasing awareness of sleep health and early diagnosis is anticipated to fuel the segmental growth. The segment is expected to exceed USD 3.3 billion by 2035, growing at a CAGR of 11.7% during the forecast period.
- HSAT devices that fall into the type 3 category are typically the most commonly used devices across the HSAT spectrum. They monitor 4-7 different parameters, including airflow, respiratory effort, oxygen saturation, and heart rate (HR).
- Additionally, type 3 devices provide the best combination of diagnostic accuracy and ease of use; hence, they are well suited to the detection of moderate to severe obstructive sleep apnea (OSA) in cases that have no major complications.
- Further, type 3 devices are among the lightest, simplest to use, and least expensive types of HSAT devices available in the market. Lastly, telehealth integration and cloud-based access for remote review by a physician have added to the appeal of Type 3 HSAT devices.
- The type 2 segment was valued at USD 145.3 million in 2025. A type 2 home sleep apnea test device is a portable system that mimics an in-lab polysomnographic test. It records the same parameters as other polysomnographic tests, including EEG, EOG, EMG, airflow, respiratory effort, SpO2, and heart rate.
- The device provides a full range of data useful for diagnosing complicated sleep disorders (e.g., complicated sleep apnea with co-morbidities such as cardiovascular, neurological conditions, etc.).
- In order to achieve accurate results, Type 2 devices must be professionally set up and interpreted, therefore making these devices less convenient than other HSAT device types.
- The type 4 segment was valued at USD 394.6 million in 2025. Devices classified as Type 4 simplified monitors may primarily track around 1–2 parameters, most commonly oxygen saturation and/or heart rate. They are portable and provide a low-cost means for patient convenience and initial assessments or large-scale population studies.
- These devices offer consumers and healthcare providers a quick and easy way to evaluate health/cardiac needs, but the limitations of the measured data may lead to inaccurate diagnoses due to the need for extensive follow-up tests with more sophisticated monitoring devices.
- Moreover, the low price and the integration of these devices with mobile health applications encourage patients to utilize them, propelling the segmental growth.
Based on indication, the global home sleep apnea testing devices market is segmented into obstructive sleep apnea (OSA) and central sleep apnea. The obstructive sleep apnea segment accounted for a dominating share and was valued at USD 1.5 billion in 2025.
- Obstructive sleep apnea (OSA) is the most prevalent type of sleep apnea that occurs when the airway gets blocked while sleeping, resulting in multiple pauses in breathing and a decrease in oxygen level.
- OSA is directly related to obesity and increases the risk of developing high blood pressure, heart conditions, and diabetes; therefore, the early diagnosis of OSA is critically important.
- Further, home sleep apnea testing devices (HSAT) have become one of the preferred and commonly utilized methods of diagnosing OSA since they are effective at measuring airflow, respiratory effort, and oxygen saturation and at the same time provide patients with a convenient home testing option at an affordable rate compared with in-lab studies. Thus, the growing number of OSA patients and increased awareness and understanding of the disorder are anticipated to fuel the industry's growth over the analysis period.
- The central sleep apnea segment accounted for a revenue of USD 74.1 million in 2025. In central sleep apnea (CSA), the brain malfunctions to provide normal signals to the lungs for breathing, leading to cessation of breathing or periodic emergency stops. There are many potential causes, including cardiac failure, neurological disorders, and the use of opioids.
- However, HSAT may act as a preliminary screening platform, which may identify certain conditions, but for proper evaluation of CSA in patients, laboratory-based tests are necessary.
Learn more about the key segments shaping this market
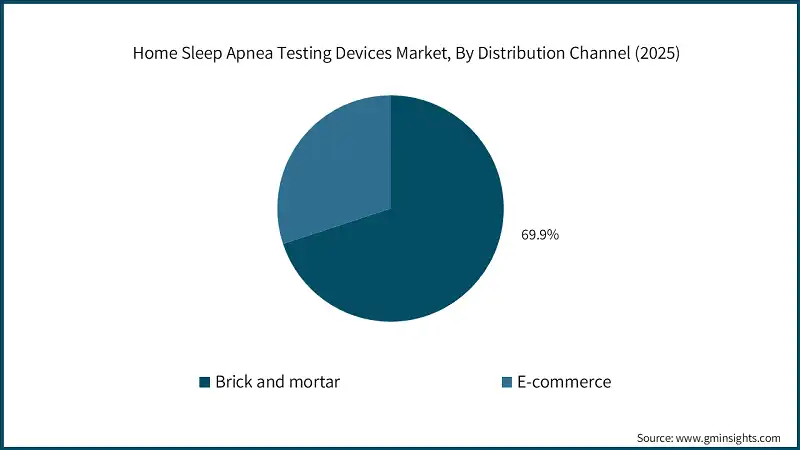
Based on distribution channel, the home sleep apnea testing devices market is segmented into brick and mortar, and E-commerce. The brick and mortar segment accounted for the leading market share of 69.9% in 2025.
- HSAT’s brick-and-mortar or offline channels consist of hospitals, sleep clinics, DME suppliers, and retail pharmacies. These channels play a critical role for home sleep apnea testing devices because a clinician generally prescribes, fits, and educates patients on how to use their HSAT device, thereby reducing errors and increasing patient compliance.
- Additionally, the presence of brick-and-mortar locations allows patients to verify their insurance coverage, rent devices from DME suppliers, and establish a chain of custody for their devices. Brick-and-mortar locations also offer services related to troubleshooting devices or sensors, replacing worn or defective parts, and providing post-test follow-up care.
- Further, brick-and-mortar channels may have slower growth than online channels, but they tend to excel in cases that require more complexities, as well as for cases that are handled through institutional contracts, thus supporting the industry growth.
- On the other hand, the e-commerce segment is expected to record higher growth with a 12.1% CAGR over the forecast period. E-commerce includes all telehealth platforms, vendor websites, and distributors that support the shipment of Home Sleep Apnea Testing (HSAT) kits through mobile applications.
- E-commerce models simplify the process of screening for eligibility, obtaining consent, and managing logistics; therefore, patients receive their devices through the mail, perform the test in their home, and transmit their data to secure encrypted websites provided by healthcare providers for review.
- Furthermore, e-commerce channels offer increased access to underserved populations, reduce waiting time, and also provide opportunities for multi-night testing packages and subscription services.
- Moreover, digital education, chat support, and automatic reminders promote patient compliance, while transparent pricing promotes payment options for uninsured patients.
Looking for region specific data?
North America Home Sleep Apnea Testing Devices Market
North America market accounted for majority share of 33.6% in 2025 in the global market and is anticipated to show notable growth over the forecast period.
- The HSAT market is dominated by North America due to a substantial increase in consumers awareness related to the effects of sleep disorders, the presence of advanced healthcare resources, and the high rate of telehealth utilization. The North American market has a high prevalence of OSA associated with obesity-related issues, lifestyle choices, and other conditions. For example, according to the data published in a recent article, over 263,000 children undergo tonsillectomies per year in the U.S. In the majority of cases, the operations are performed due to the presence of sleep apnea in the children that is caused by the tonsils obstructing their airway.
- The reimbursement policy related to health insurance for home sleep testing has fostered a greater acceptance of home sleep testing due to improved digital health integration and improved access.
- An increased interest in remote monitoring and subscription models is indicative of consumers preference for convenience. There are clinical guidelines supporting the adoption of HSAT in uncomplicated cases, contributing to market growth.
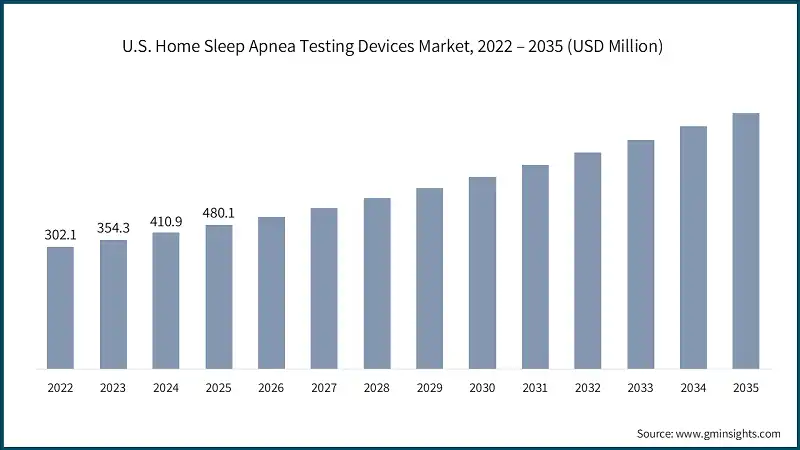
The U.S. home sleep apnea testing devices market was valued at USD 302.1 million and USD 354.3 million in 2022 and 2023, respectively. In 2025 the market size was valued at USD 480.1 million from USD 410.9 million in 2024. Increasing demand for connected healthcare solutions and subscription-based models for HSAT services is projected to fuel the market growth.
- The U.S. accounts for the largest market share of home sleep apnea testing (HSAT) among all countries due to numerous factors such as high incidence rates of obstructive sleep apnea (OSA), rising awareness about sleep health, and well-established telemedicine systems.
- The adoption of HSAT in practice has been supported by favorable reimbursement policies and acceptance of HSAT by physicians. In response to rising healthcare costs, patients are shifting towards utilizing low-cost alternatives such as at-home testing. HSATs have a significantly lower cost in comparison to laboratory-based procedures.
- In addition, the rise in the number of digital platforms is playing a key role in shipping the product, and sharing test data has considerably contributed towards this change.
- The advancement of technology has helped to further improve the way in which HSAT results are scored via artificial intelligence and smartphones, as well as providing the ability for all patients to adhere to the recommended treatment for OSA.
Europe Home Sleep Apnea Testing Devices Market
Europe accounted for a significant share of the global market and was valued at USD 404 million in 2025.
- Europe continues to have a steady increase in HSAT use due to the push for digital health through government programs and increased awareness. Countries such as Germany, France, and the UK have strong healthcare systems and are at the forefront of implementing HSATs.
- Reimbursement schemes vary across different countries in Europe. Integration of HSAT devices with telehealth solutions has gained popularity, primarily within urban regions.
- The industry has witnessed substantial growth due to technology advancements and collaborations with device manufacturers and sleep clinics.
- Additionally, rising numbers of older adults and cases of cardiovascular disease are anticipated to rise, pushing the need for sleep apnea devices, thus fueling the market growth.
Germany held significant share of the European home sleep apnea testing devices market, showcasing strong growth potential.
- Germany represents a large market for home sleep apnea testing devices due to its well-developed healthcare system combined with a high burden of sleep disorders. Strong emphasis on clinical accuracy and adherence to strict regulatory standards influences device selection.
- Hospitals and sleep clinics currently serve as the primary venues for distribution of these devices; however, utilizing telehealth is a growing trend to reach patients.
- Additionally, an aging population along with a growing obesity epidemic is creating an increased demand for home sleep apnea testing devices throughout the country.
- Further, an increase in the use of electronic health records and cloud-based systems is enhancing workflow productivity through increased efficiency.
Asia Pacific Home Sleep Apnea Testing Devices Market
The Asia Pacific market accounted for a substantial share of the market and was valued at USD 376.3 million in 2025.
- The rapid growth of the HSAT market in the Asia Pacific region is supported by the improved healthcare infrastructure and disposable income levels, as well as rising awareness of the importance of sleep health.
- In addition, urbanization and changing lifestyles have resulted in the increased prevalence of sleep apnea, especially in countries such as China and India.
- Local manufacturers are introducing cost-effective solutions to cater to price-sensitive markets.
- Additionally, government programs that support digital health and remote patient monitoring continue to promote growth in the market.
China home sleep apnea testing devices market is estimated to grow with a robust CAGR in the Asia Pacific market.
- HSATs represent a significant growth opportunity in China due to its large population and an increasing prevalence of sleep apnea, especially in the urban population due to sedentary lifestyle disorders such as obesity and other conditions.
- In addition, rapid digital transformation and widespread use of smartphones are anticipated to enable telehealth integration, resulting in increased popularity of home testing. Domestic companies are entering the market with low-cost, portable devices, which adds to the competitive landscape.
- Additionally, government policies are encouraging the use of remote healthcare to manage chronic diseases and increase usage of HSATs. Moreover, e-commerce is expected to increasingly become a preferred channel for distributing the HSAT device, providing greater convenience and lower prices for consumers.
Latin America Home Sleep Apnea Testing Devices Market
Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period.
- Brazil’s HSAT market is expanding as awareness of sleep disorders grows and telemedicine adoption accelerates. Economic constraints make cost-effective home testing attractive compared to in-lab studies.
- Urban regions lead in adoption, supported by private healthcare providers and partnerships with device manufacturers. E-commerce channels are gaining traction, offering HSAT kits with remote physician review.
- Additionally, the rising prevalence of obesity and cardiovascular diseases ensures sustained demand for testing kits.
- Further, technological advancements and localized manufacturing are expected to further drive growth in the coming years.
Middle East and Africa Home Sleep Apnea Testing Devices Market
Saudi Arabia market to experience substantial growth in the Middle East and Africa market in 2025.
- Saudi Arabia is witnessing increasing home sleep apnea testing device adoption, driven by rising prevalence of sleep apnea and government initiatives promoting digital health under Vision 2030.
- In addition, high smartphone penetration and telemedicine platforms enable remote diagnostics, aligning with patient preference for convenience. Private healthcare providers and specialty clinics are key distribution channels, while e-commerce is emerging as a preferred alternative.
- Further, cultural emphasis on premium healthcare services supports uptake of advanced HSAT devices. Moreover, growing investment in healthcare infrastructure and chronic disease management programs is anticipated to continue to fuel market growth.
Home Sleep Apnea Testing Devices Market Share
The market for home sleep apnea testing (HSAT) is moderately consolidated, with both global players and niche innovators competing against each other based on technology, connectivity, and service integration. Both ResMed and Philips have established themselves as industry leaders through strong brand recognition and extensive product portfolios and by developing cloud platforms capable of telemonitoring and data analysis.
Other key players (e.g., Nihon Kohden and Natus) are focused on clinical accuracy and hospital integration, serving institutional buyers. Whereas, ZOLL Itamar has positioned itself as an innovator through a wearable HSAT solution (WatchPAT), which emphasizes the comfort of the patient and is supported by AI-based algorithms for diagnosis.
Emerging companies and regional manufacturers compete on price and mobile health integration in price-sensitive markets. These businesses view strategic collaborations with telehealth providers, subscription models, and AI algorithm development as primary means to fuel growth going forward. Furthermore, as companies invest in miniaturizing devices, extending testing periods to multiple nights, and creating interoperability with electronic health records, the competitive environment for HSAT devices is likely to become increasingly intensive in the future.
Home Sleep Apnea Testing Devices Market Companies
A few of the prominent players operating in the global home sleep apnea testing devices industry include:
- CADWELL
- CleveMed
- COMPUMEDICS
- CONTEC
- natus
- Neurosoft
- NEUROVIRTUAL
- NIHON KOHDEN
- nox MEDICAL
- PHILIPS
- Resmed
- SOMNOmedics
- ZOLL itamar
- Resmed
ResMed focuses on expanding its connected health ecosystem through cloud-based platforms and telemonitoring solutions. Invests heavily in R&D for AI-driven diagnostics and wearable HSAT devices while pursuing global market penetration via strategic partnerships and acquisitions.
Nihon Kohden targets technological differentiation by developing advanced diagnostic sensors and portable monitoring systems. Emphasizes clinical accuracy and reliability while expanding presence in emerging markets through localized manufacturing and strategic alliances with healthcare providers.
ZOLL Itamar specializes in wearable HSAT solutions like WatchPAT, emphasizing patient comfort and ease of use. Pursues growth through partnerships with telemedicine providers and insurance companies while advancing AI-based analytics for personalized sleep disorder management.
Home Sleep Apnea Testing Devices Industry News:
- In September 2025, SOMNOmedics announced that the U.S. Food and Drug Administration (FDA) had provided clearance to its two versions of a new Type II home sleep test for detecting sleep-related breathing disorders, sleep staging, and measuring snoring levels. The HomeSleepTest and HomeSleepTest REM+ can have use cases ranging from insomnia screening to CPAP efficacy monitoring. This development may help the company in product expansion with a focus on improving business prospects.
- In April 2025, Resmed announced its home sleep apnea test, NightOwl, available across the U.S. It is an FDA-approved home sleep apnea test (HSAT) designed to provide healthcare providers with a simplified, accurate, and efficient way to diagnose obstructive sleep apnea (OSA) from the comfort of an individual’s home. This development may enable the company to further consolidate its industry position in the U.S. market.
- In December 2021, Zoll Medical Corporation reported that it had completed the previously announced acquisition of Itamar Medical Ltd., a medical device and digital health company that provides at-home testing for sleep apnea. This acquisition enabled the company to gain considerable presence in the consistently growing HSAT industry.
The global home sleep apnea testing devices market research report includes an in-depth coverage of the industry with estimates and forecasts in terms of revenue in (USD Million) from 2022 - 2035 for the following segments:
Market, By Test Type
- Type 2
- Type 3
- Type 4
Market, By Indication
- Obstructive sleep apnea (OSA)
- Central sleep apnea
Market, By Distribution Channel
- Brick and mortar
- E-commerce
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- MEA
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which distribution channel led the HSAT market in 2025?
The brick-and-mortar segment held 69.9% market share in 2025, driven by hospital-based prescriptions, DME supplier networks, and clinician-led device fitting and guidance.
Which region led the global HSAT market in 2025?
North America led the market with a 33.6% share in 2025, supported by high OSA prevalence, advanced telehealth systems, and favorable reimbursement for home sleep testing.
Which indication accounted for the largest share in 2025?
Obstructive Sleep Apnea (OSA) dominated the market with USD 1.5 billion revenue in 2025, due to its high global prevalence and increasing need for early, convenient diagnosis.
What was the valuation of the Type 2 HSAT segment in 2025?
The Type 2 segment was valued at USD 145.3 million in 2025, reflecting its use in complex sleep disorder diagnosis with near-polysomnography-level measurement capabilities.
How much revenue did the Type 4 HSAT segment generate in 2025?
The Type 4 segment generated USD 394.6 million in 2025, driven by affordability and adoption in large-scale screening and primary assessments.
Who are the key players in the home sleep apnea testing devices market?
Leading players include ResMed, Philips, Nihon Kohden, Natus, ZOLL Itamar, CleveMed, Compumedics, Nox Medical, Neurosoft, and SOMNOmedics. The top five players collectively account for ~60% market share.
Which test type segment held the largest share in 2025?
The Type 3 segment led the market with a 66.1% share in 2025, owing to its balance of diagnostic accuracy, cost-effectiveness, and telehealth integration.
What is the projected value of the home sleep apnea testing devices market by 2035?
The market is forecast to grow to USD 5 billion by 2035, progressing at a strong 11.6% CAGR during 2026–2035.
What is the estimated market size of the HSAT devices market in 2026?
The market is expected to reach USD 1.9 billion in 2026, supported by technological upgrades, wider telemedicine adoption, and increasing awareness of sleep health.
What was the market size of the home sleep apnea testing devices market in 2025?
The global market for home sleep apnea testing devices was valued at USD 1.6 billion in 2025, driven by rising sleep apnea prevalence and strong demand for convenient home-based testing solutions.
Home Sleep Apnea Testing Devices Market Scope
Related Reports


