Summary
Table of Content

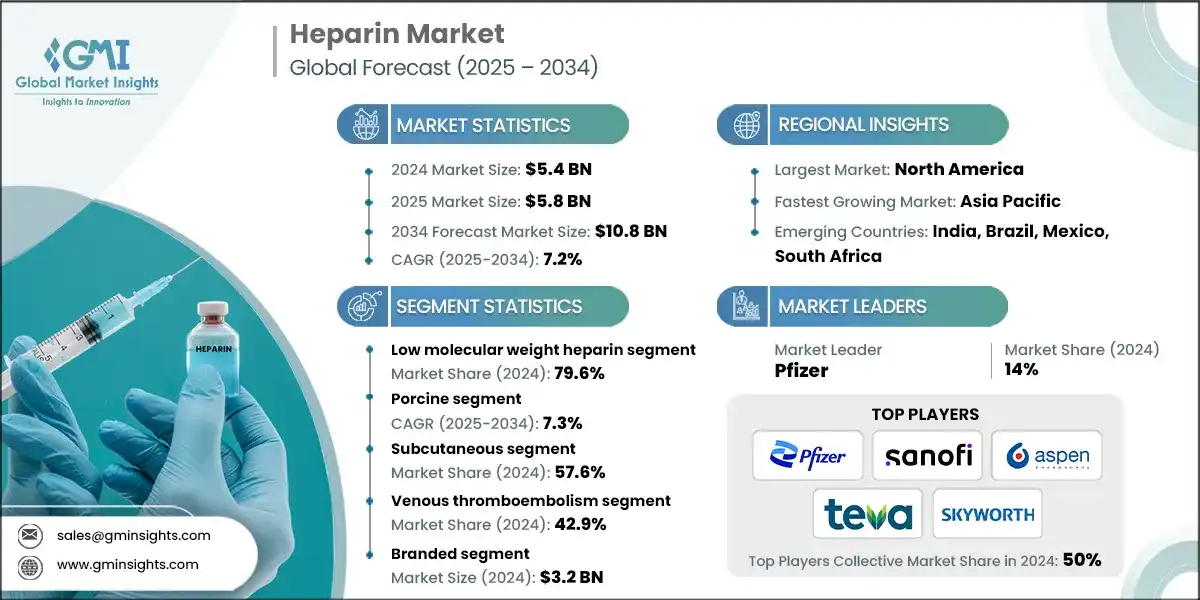
Heparin Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Heparin Market Size
The global heparin market was estimated at USD 5.4 billion in 2024. The market is expected to grow from USD 5.8 billion in 2025 to USD 10.8 billion in 2034, at a CAGR of 7.2%, according to the latest report published by Global Market Insights Inc. This growth is driven by the increasing prevalence of cardiovascular and thrombotic disorders, a rise in surgical procedures, and expanding applications in dialysis, oncology, and medical devices.
Heparin Market Key Takeaways
Market Size & Growth
- 2024 Market Size: USD 5.4 Billion
- 2025 Market Size: USD 5.8 Billion
- 2034 Forecast Market Size: USD 10.8 Billion
- CAGR (2025–2034): 7.2%
Regional Dominance
- Largest Market: North America
- Fastest Growing Region: Asia Pacific
Key Market Drivers
- Rising global cardiovascular disease prevalence.
- Aging population and surgical procedures.
- Expansion of hospital infrastructure and homecare.
- Government and institutional health initiatives.
Challenges
- High cost of branded LMWH and biosimilars.
- Supply chain disruptions and raw material dependency.
Opportunity
- Development of synthetic and recombinant heparin.
- Emerging markets with rising healthcare investment.
Key Players
- Market Leader: Pfizer led with over 14% market share in 2024.
- Leading Players: Top 5 players in this market include Pfizer, Sanofi, Aspen Pharmacare, Teva Pharmaceutical Industries, Shenzhen Hepalink Pharmaceuticals, which collectively held a market share of 50% in 2024.
Get Market Insights & Growth Opportunities
According to the World Health Organization (WHO), cardiovascular diseases are among the leading causes of death globally, accounting for an estimated 17.9 million deaths annually. Furthermore, the American Heart Association reports that thromboembolic condition is responsible for one in every four deaths worldwide. These alarming statistics underscore the urgent need for public health awareness, thereby fueling the demand for venous thromboembolism treatment.
The heparin market is referred to as the pharmaceutical segment that is focused on anticoagulants therapy used to prevent and treat blood clots in conditions such as deep vein thrombosis, pulmonary embolism, and atrial fibrillation. It includes products such as unfractionated heparin, low molecular weight heparin, and ultra-low molecular weight heparin, administered primarily through injectable solutions and pre-filled syringes.
Major industry players such as Pfizer, Sanofi, Shenzhen Hepalink Pharmaceuticals, Fresenius Kabi, and Leo Pharma dominate the market through continuous product innovation and robust global distribution strategies.
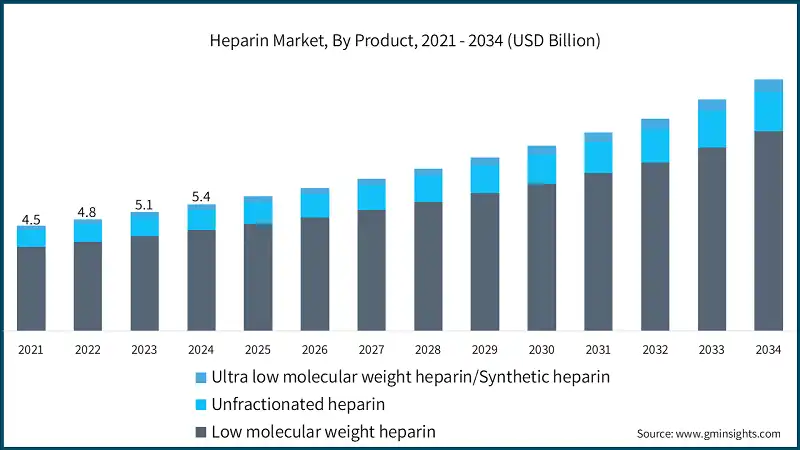
The market has shown consistent growth, rising from USD 4.5 billion in 2021 to USD 5.1 billion in 2023, driven by increased healthcare spending, regulatory approvals, and innovations in biosynthetic and microbial heparin production. The growing adoption of low weight heparin due to its improved safety and ease of administration is reshaping treatment protocols. Moreover, the introduction of safety syringes, needle-free injectors, and advanced drug delivery systems is improving patient compliance and minimizing adverse events. Government initiatives and strategic collaborations such as national anticoagulation programs and partnerships for biosimilar development are further propelling market growth.
The market is further strengthened by rising investments in healthcare infrastructure, particularly in developing regions such as Asia-Pacific and Latin America. The heparin market is poised for significant growth due to increasing research and development efforts aimed at creating novel anticoagulants with enhanced efficacy and reduced side effects. These factors are expected to boost the adoption of heparin-based therapies across hospitals, clinics, and homecare settings in the coming years. Thus, the growing burden of cardiovascular diseases, coupled with advancements in anticoagulant technologies, is fostering robust growth within the market.
To get key market trends
Heparin Market Trends
- The global market is undergoing dynamic transformation, driven by the rising prevalence of cardiovascular diseases, increasing surgical procedures and expanding use in dialysis, oncology, and critical care settings. This baseline demand is further supported by aging populations and growing awareness of thrombotic disorders across both developed and emerging regions.
- Additionally, the shift toward premium biosimilar and synthetic formulations is gaining momentum as healthcare systems increasingly prioritize cost-effective yet high-quality alternatives. For example, low molecular weight heparins such as enoxaparin and dalteparin are becoming more widely adopted due to their predictable pharmacokinetics and reduced need for monitoring. This trend is driving innovation in prefilled syringes, autoinjectors, and safety-engineered delivery systems, all aimed at enhancing patient comfort and compliance.
- Moreover, large-scale public health initiatives such as national anticoagulation programs and hospital-based thrombosis prevention campaigns are driving periodic bulk procurement of heparin products. These programs significantly influence production planning and distribution strategies for manufacturers and suppliers.
- The market is also witnessing an increasing shift toward personalized anticoagulant therapy, especially in high-risk populations such as cancer patients, post-operative cases, and individuals with atrial fibrillation drugs. This has created demand for advanced formulations such as ultra-low molecular weight heparins (ULMWHs) and smart infusion systems that offer precise dosing and reduced side effects, aligning with the rise of biologics and specialty drugs in thrombosis care.
- A growing shortage of specialized healthcare professionals, particularly in rural and underserved areas, is prompting demand for simplified, user-friendly heparin delivery systems. Prefilled syringes, fixed-dose autoinjectors, and needle-free devices are helping optimize workflows and reduce dependency on specialist supervision.
- Hospitals and outpatient centers are increasingly seeking heparin products that can be safely administered by non-specialist staff. Manual dosing is often error-prone and time-consuming, particularly in high-volume clinical settings. To address these challenges, manufacturers are developing automated infusion pumps, multi-dose delivery systems, and integrated safety mechanisms designed to prevent overdosing and ensure consistent therapeutic outcomes.
Heparin Market Analysis
Learn more about the key segments shaping this market
Based on product, the heparin market is categorized into low molecular weight heparin, unfractionated heparin and ultra-low molecular weight heparin/synthetic heparin. The low molecular weight heparin segment accounted for 79.6% of the market in 2024 which is stimulated by superior pharmacokinetic profile, reduced risk of bleeding complications, ease of administration, widespread clinical adoption, and growing preference for outpatient treatment settings. The segment is expected to exceed USD 8.6 billion by 2034, growing at a CAGR of 7.2% during the forecast period.
- The low molecular weight heparin (LMWH) segment leads the heparin market, primarily due to its favorable pharmacokinetic profile, including a predictable anticoagulant response and longer half-life, which reduce the need for frequent dosing and monitoring.
- LMWHs are preferred in clinical settings for the treatment of venous thromboembolism, owing to their lower bleeding risk and improved safety outcomes. According to Lippincott, LMWHs are associated with a reduced risk of venous thromboembolism recurrence, fewer bleeding complications, and lower 30-day mortality rates compared to unfractionated heparin.
- Additionally, their suitability for subcutaneous administration and lower risk of heparin-induced thrombocytopenia (HIT) make them ideal for long-term use, especially in high-risk populations such as pregnant women and cancer patients.
- The ultra-low molecular weight heparin/synthetic heparin segment is projected to grow at a CAGR of 8.7%, driven by innovations in synthetic anticoagulants that offer enhanced bioavailability and reduced immunogenicity. The expansion of this segment is supported by ongoing research in targeted drug delivery and the development of next-generation anticoagulants for niche therapeutic applications.
- The unfractionated heparin segment is expected to grow at a CAGR of 6.9%. The growth of this segment is due to its critical role in acute care settings, especially during cardiovascular surgeries and dialysis procedures.
Based on source, the heparin market is segmented into porcine and bovine. The porcine segment accounted for the significant CAGR of 7.3% in 2024.
- This segment is propelled by the widespread availability, consistent quality, and long-standing clinical use of porcine-derived heparin. Porcine mucosal heparin is the primary global source of raw material for heparin production and the exclusive source for the most widely used low molecular weight heparin, enoxaparin.
- Additionally, porcine-derived heparin is associated with a lower risk of heparin-induced thrombocytopenia (HIT), a serious complication affecting many patients undergoing cardiovascular surgeries. As noted in The Annals of Thoracic Surgery, bovine heparin is believed to carry a higher risk of HIT compared to porcine heparin, further reinforcing the market preference for porcine-based formulations.
- On the other hand, the bovine-derived heparin segment is expected to experience steady growth, driven by efforts to diversify sourcing and reduce reliance on porcine materials.
- Bovine heparin is gaining traction in regions where cultural or religious restrictions limit the use of porcine products. Its reintroduction into regulated markets is supported by enhanced safety protocols and clinical equivalence studies, which demonstrate its efficacy and safety profile comparable to porcine-derived alternatives.
Based on route of administration, the heparin market is segmented into intravenous and subcutaneous. The subcutaneous segment accounted for the highest market share of 57.6% in 2024, the segment domination is stimulated by its convenience and a smaller number of hospital visits, along with suitability for long-term anticoagulant therapy.
- Subcutaneous heparin is preferred in post-operative care and obstetrics, that requires continuous anticoagulation without intensive monitoring. For instance, Science Direct mentions, the subcutaneous route of administration offers decreased time and convenient alternative for therapeutic agents such as heparin.
- Additionally, new technologies that includes needle technology and stabilization for formulation have enhanced patient safety and efficacy, that contributes to the stimulating segment demand, further escalating the market demand.
- This route of administration is generally preferred for low molecular weight heparin type, particularly in outpatient and chronic care. The subcutaneous segment allows for self-administration with minor training from healthcare providers, which improves patient compliance and reduces healthcare costs associated with intravenous infusions.
- The intravenous segment is expected to maintain steady growth, escalated by its critical role in acute care scenarios such as cardiac surgeries, dialysis, and intensive care units. Intravenous administration allows for rapid onset of action and precise dose control, making it essential in emergency settings.
Based on application, the heparin market is segmented into venous thromboembolism, atrial fibrillation/flutter, coronary artery disease and other applications. The venous thromboembolism segment accounted for the highest market share of 42.9% in 2024 due to the higher global incidences and widespread use of heparin in both prophylactic and therapeutic settings.
- The segment is driven by the clinical urgency, venous thromboembolism includes deep vein thrombosis and pulmonary embolism, remains a major cause of morbidity and mortality, especially among hospitalized and post-surgical patients. According to American Heart Association Journals, venous thrombosis is a major public health priority, that causes 1 in 4 deaths globally.
- Additionally, heparin is the first-line treatment for this disease, low molecular weight heparin is particularly preferred due to its rapid action and proven efficacy.
- Moreover, technological advancements such as biosimilar low molecular weight, extended-release formulations, and digital adherence tools have enhanced the management of venous thromboembolism. These innovations support better patient outcomes and reduce recurrence rates, while also improving cost-efficiency for healthcare systems.
- The segment is further benefited from strong clinical evidence, broad insurance coverage, and increasing awareness of venous thromboembolism risks among healthcare providers.
- On the other hand, the coronary artery disease segment is expected to grow steadily, driven by the rising prevalence of cardiovascular conditions and the critical role of heparin in interventional procedures such as angioplasty and stent placement.
- The atrial fibrillation/flutter segment is projected to expand due to the growing burden of arrhythmias and the need for stroke prevention in affected patients. Heparin is frequently used as a bridging therapy during initiation or interruption of oral anticoagulants, especially in perioperative settings.
Based on type, the heparin market is segmented into branded and generics. The branded segment generated the revenue of USD 3.2 billion in 2024 due to the higher patient trust and compliance in branded products.
- The branded heparin segment continues to thrive, supported by the reliability of clinical trials, regulatory approvals, and physician preference for trusted therapeutic solutions. These branded drugs have undergone extensive clinical testing and post-marketing surveillance, ensuring their efficacy and safety particularly in critical care settings where precision and predictable outcomes are essential.
- Branded heparins are widely adopted by hospital formularies, government institutions, and national health programs, backed by robust global supply chains and distribution networks. Their availability in advanced drug delivery formats, such as prefilled syringes and autoinjectors, further enhances patient convenience and compliance.
- Additionally, pharmaceutical companies actively invest in innovation and lifecycle management strategies to maintain brand loyalty and market share.
- On the other hand, the generics segment is poised for rapid growth, driven by cost-effectiveness, patent expirations, and increasing access in emerging markets. Generic heparins offer comparable therapeutic outcomes at significantly lower prices, making them attractive for large-scale procurement and insurance reimbursement programs.
- This segment also benefits from growing regulatory support for biosimilars and rising demand for affordable anticoagulant therapies.
Learn more about the key segments shaping this market
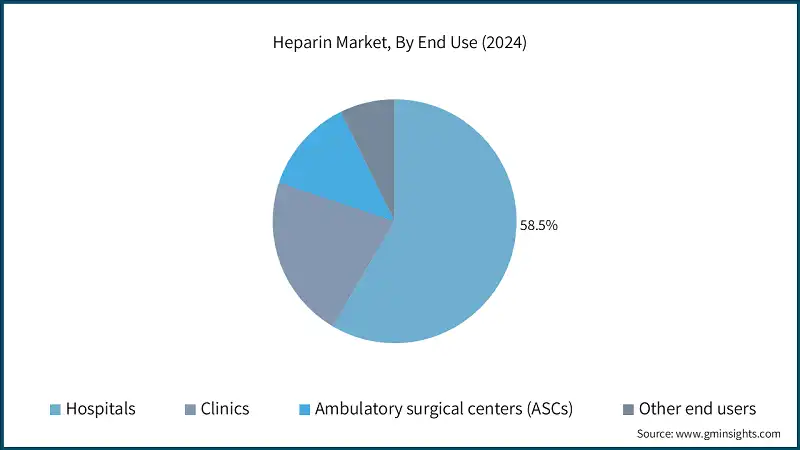
Based on end use, the heparin market is categorized into hospitals, clinics, ambulatory surgical centers (ASCs) and other end users. The segment hospitals accounted for the highest market share of 58.5% in 2024 due to easy availability and convenience, hospitals are a primary channel in the market.
- Hospitals dominate the heparin market due to their high patient volumes, extensive use of anticoagulants in surgical and emergency care, and access to advanced treatment protocols.
- Heparin plays a critical role in hospital-based procedures such as cardiac surgeries, dialysis, and thromboprophylaxis for immobile patients. Increasingly, hospitals are integrating heparin administration into electronic health records and clinical decision support systems to ensure accurate dosing and minimize adverse events. The availability of both intravenous and subcutaneous formulations allows for flexible administration tailored to patient needs.
- Additionally, hospitals benefit from bulk procurement and strong supplier relationships, ensuring consistent availability and cost efficiency.
- Meanwhile, clinics are expanding their use of heparin, particularly in chronic care management and follow-up treatments. Subcutaneous heparin is commonly administered for conditions such as venous thromboembolism and atrial fibrillation, especially for patients transitioning from hospital to home care.
- Ambulatory surgical centers (ASCs) are also witnessing steady growth in heparin usage, driven by the increasing number of outpatient procedures and the need for perioperative anticoagulation. ASCs favor low molecular weight heparins due to their ease of administration and reduced monitoring requirements.
Looking for region specific data?
North America Heparin Market
The North America market dominated the global market with a market share of 56.1% in 2024. The market is stimulated by the rising prevalence of cardiovascular diseases and increased investment in susceptible populations.
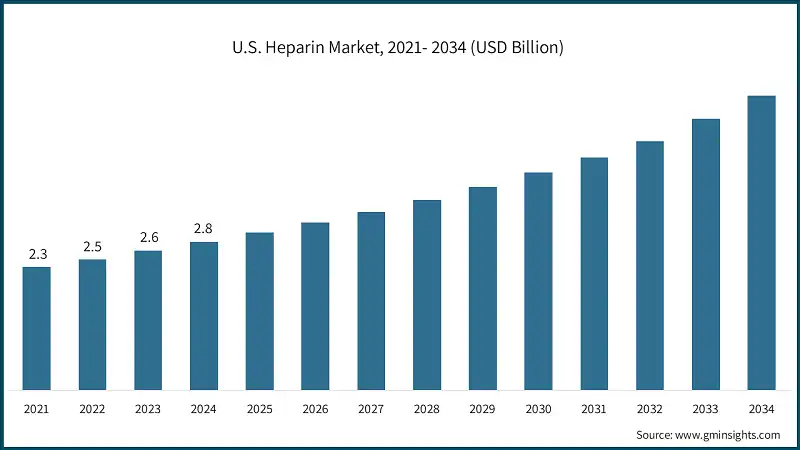
The U.S. heparin market was valued at USD 2.3 billion and USD 2.5 billion in 2021 and 2022, respectively. The market size reached USD 2.8 billion in 2024, growing from USD 2.6 billion in 2023.
- The U.S. has a high number of cases for cardiovascular surgeries and chronic disease cases, especially that involves venous thromboembolism making it a major consumer of heparin-based anticoagulants. According to NIH, more than 200,000 people develop venous thrombosis in U.S., with 50,000 cases complicated by pulmonary embolism.
- Moreover, FDA regulatory that support disease drug production and reimbursement policies accelerate the approval and adoption of both branded and generic heparin products.
- Additionally, hospitals and homecare settings that are used for prefilled syringes and autoinjectors, supporting safe and efficient anticoagulant therapy.
- Strategic stockpiling and emergency preparedness programs ensure stable heparin supply during public health crises.
- The U.S. market benefits from advanced manufacturing infrastructure, enabling rapid scale-up and innovation in heparin production.
Europe Heparin Market
Europe market accounted for USD 1.1 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- The rising incidence of venous thromboembolism and atrial fibrillation—particularly among aging populations in countries like Germany, France, and Italy is driving sustained demand for both low molecular weight heparin and unfractionated heparin therapies across inpatient and outpatient settings.
- Simultaneously, increasing regulatory scrutiny of animal-derived pharmaceuticals is accelerating the shift toward synthetic and recombinant heparin alternatives, especially in regions with religious or ethical concerns regarding porcine-sourced products.
- Additionally, the growing adoption of biosimilars is reshaping procurement strategies, as national health systems in countries such as the UK and the Nordics prioritize cost-effective LMWH generics to manage public healthcare budgets efficiently.
Germany heparin market is anticipated to witness considerable growth over the analysis period.
- In Germany, the rising prevalence of cardiovascular diseases is significantly increasing market demand for anticoagulant therapies such as heparin. According to the National Institutes of Health (NIH), over 3.7% of women and more than 6% of men in Germany suffer from coronary heart disease, particularly among aging populations with chronic conditions.
- This trend is driving the use of both low molecular weight and unfractionated heparin across various care settings. The growing emphasis on preventive healthcare supported by national cardiology guidelines and early screening programs is further escalating the use of heparin in managing stroke risk, atrial fibrillation, and thromboembolic disorders.
- Germany’s well-established healthcare infrastructure supports the recommendation and adoption of heparin for targeted drug therapies. In addition, the country’s robust pharmaceutical and hospital network including specialized clinics, pharmacies, and digital health platforms ensures wide availability and accessibility of heparin treatments.
Asia Pacific Heparin Market
The Asia Pacific market is anticipated to grow at the highest CAGR of 7.5% during the analysis timeframe.
- Key markets in the Asia-Pacific region including China, India, Japan, South Korea, and Australia are witnessing rising demand for advanced heparin-based therapeutics, driven by the increasing prevalence of cardiovascular diseases and growing healthcare investments.
- The expansion of hospitals, diagnostic centers, and specialty care clinics, particularly in urban areas, is further stimulating the growth of the market. These developments are enhancing access to anticoagulant therapies and supporting broader adoption across both inpatient and outpatient settings.
China heparin market is predicted to grow significantly over the forecast period.
- China has a high rapid urbanization and healthcare modernization in the country is contributing to a rise in cardiovascular disease cases. According to NIH, about 330 million individuals in China experience cardiovascular diseases, with 13 million strokes, 5 million pulmonary heart disease, 4.87 million atrial fibrillation of it, stimulating the market demand.
- Additionally, expanding healthcare infrastructure and insurance coverage in Tier 2 and Tier 3 cities is increasing access to injectable anticoagulants, especially LMWH, for outpatient and chronic care settings.
- Moreover, the government is investing in healthcare infrastructure and digital platforms to improve distribution and treatment access across urban and rural areas.
Latin America Heparin Market
Brazil is experiencing significant growth in the Latin America market due to the increasing demand for thrombosis solutions and long-term care.
- In Brazil, government-led initiatives are strengthening cardiovascular care through national anticoagulation programs and standardized public hospital protocols, driving consistent demand for heparin therapies in stroke and thrombosis management.
- The country relies heavily on injectable anticoagulants, particularly low molecular weight heparin (LMWH), to manage surgical and post-operative complications. The widespread use of LMWH in public hospitals is fueling demand for advanced delivery formats such as prefilled syringes and autoinjectors, contributing to overall market growth.
Middle East and Africa Heparin Market
Saudi Arabia market is poised to witness substantial growth in Middle East and Africa market during the forecast period.
- The government’s Vision 2030 is driving expansion of cardiovascular care infrastructure, including specialized clinics, mobile health units, and diagnostic labs. National programs for stroke and thrombosis prevention reflect strong policy support for anticoagulant therapies, contributing to market growth.
- Rising disposable income and evolving health awareness have led to increased demand for chronic disease management, including anticoagulant therapy for conditions like atrial fibrillation and deep vein thrombosis, boosting the use of injectable heparin products.
Heparin Market Share
The heparin market is shaped by a mix of established global leaders and specialized manufacturers, creating a dynamic and moderately consolidated competitive landscape. Key players such as Pfizer, Sanofi, Aspen Pharmacare, Teva Pharmaceutical Industries, and Shenzhen Hepalink Pharmaceuticals collectively account for a significant portion of the market share, estimated at around 50%.
These companies maintain their dominance through strategic investments in biosimilar development, advanced delivery systems, and regulatory compliance, while also tailoring solutions to meet the evolving needs of anticoagulant therapies, particularly in surgical, cardiovascular, and chronic care settings.
To strengthen their market positions, leading firms are adopting multi-pronged strategies including acquisitions, partnerships, and competitive pricing. These efforts aim to make heparin therapies more accessible and cost-effective, while also addressing unmet clinical needs.
In addition to these dominant players, companies such as Dr. Reddy’s Laboratories, Bioiberica, Aspen Pharmacare, and Suanfarma are contributing to the market’s growth through technological innovation, high-quality API production, and region-specific strategies. Their presence is particularly notable in Asia-Pacific and Latin America, where rising demand for anticoagulant care and expanding healthcare infrastructure are driving increased adoption of injectable heparin solutions.
Overall, the market is witnessing intensified competition and greater diversity, as both established and emerging players continue to evolve their offerings and strategies to meet the global demand for effective and efficient heparin therapies.
Heparin Market Companies
Few prominent players operating in the heparin industry includes:
- Amphastar
- Aspen Pharmacare
- Bioiberica
- Changzhou Qianhong Biopharma
- Dr. Reddy’s Laboratories
- Fresenius Kabi
- Laboratorios Farmaceuticos ROVI
- Leo Pharma
- Nanjing King-Friend Biochemical Pharmaceutical
- Pfizer
- Sanofi
- Shenzhen Hepalink Pharmaceuticals
- Suanfarma
- Teva Pharmaceutical Industries
- Yantai Dongcheng Biochemicals
- Pfizer
Pfizer leads the heparin market with a share of 14% and a strong portfolio centered; it commercializes low molecular weight heparin widely used in thrombosis prevention. Its leadership is driven by global clinical adoption, robust regulatory approvals, and innovation in injectable delivery formats.
Sanofi stands out for its expertise in LMWH therapies, with Lovenox being a flagship product in surgical and cardiovascular care. While its broader portfolio spans multiple therapeutic areas, its advanced formulations and global distribution capabilities are increasingly applied to heparin management. Sanofi’s strength lies in its innovation in safety syringes and hospital-based protocols, making it a trusted name in anticoagulant therapy.
Leo Pharma specializes in thrombosis care with Innohep, offering targeted LMWH solutions for deep vein thrombosis and cancer-associated thrombosis. With a strong research and development pipeline and strategic focus on niche indications, Leo Pharma continues to expand its heparin offerings. The company’s European reach and commitment to clinical precision position as a key player in advancing next-generation heparin therapies.
Heparin Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 5.4 Billion |
| Market Size in 2025 | USD 5.8 Billion |
| Forecast Period 2025 - 2034 CAGR | 7.2% |
| Market Size in 2034 | USD 10.8 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising global cardiovascular disease prevalence | Increasing cases of thrombosis, stroke, and heart disease are driving demand for anticoagulants. |
| Aging population and surgical procedures | Older adults undergoing surgeries and chronic treatments require heparin for clot prevention and post-operative care. |
| Expansion of hospital infrastructure and homecare | Improved access to healthcare facilities and home-based treatment options are accelerating heparin adoption. |
| Government and institutional health initiatives | National programs promoting anticoagulant therapy and safety standards are boosting awareness and early intervention. |
| Pitfalls & Challenges | Impact |
| High cost of branded LMWH and biosimilars | Limits affordability in low-income regions, affecting equitable access to advanced heparin therapies. |
| Supply chain disruptions and raw material dependency | Reliance on porcine sources and global logistics challenges can hinder consistent product availability. |
| Opportunities: | Impact |
| Development of synthetic and recombinant heparin | Novel formulations reduce dependency on animal sources and improve safety, consistency, and scalability. |
| Emerging markets with rising healthcare investment | Asia-Pacific and Latin America offer high growth potential due to expanding healthcare infrastructure and demand for anticoagulants. |
| Market Leaders (2024) | |
| Market Leaders |
14% market share |
| Top Players |
Collective Market Share is 50% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Heparin Industry News
- In July 2025, B. Braun Medical expanded its heparin sodium injection portfolio in the U.S. by launching two new premixed products: 25,000 Units in 0.45% sodium chloride injection (50 units/mL and 100 units/mL). This expansion strengthened B. Braun’s position as the provider of premixed heparin bags in the U.S. market.
- June 2023, Techdow USA launched its generic version of Lovenox (enoxaparin sodium) in prefilled syringes across seven dosage strengths. This launch strengthened its position in the U.S. generic heparin market.
- In January 2022, Optimvia and Ginkgo Bioworks partnered to improve the manufacturing efficiency of biosynthetic heparin using cell and enzyme engineering platforms. The collaboration aimed to reduce reliance on animal-derived sources and enhance scalability through fermentation-based production.
The heparin drugs market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2021 - 2034 for the following segments:
Market, By Product
- Low molecular weight heparin
- Unfractionated heparin
- Ultra-low molecular weight heparin/synthetic heparin
Market, By Source
- Porcine
- Bovine
Market, By Route of Administration
- Intravenous
- Subcutaneous
Market, By Application
- Venous thromboembolism
- Atrial fibrillation/flutter
- Coronary artery disease
- Other applications
Market, By Type
- Branded
- Generics
Market, By End Use
- Hospitals
- Clinics
- Ambulatory surgical centers (ASCs)
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which region leads the heparin market?
North America dominated the global market with a 56.1% share in 2024, supported by the rising prevalence of cardiovascular diseases and increased investment in healthcare for susceptible populations.
Who are the key players in the heparin market?
Key players include Amphastar, Aspen Pharmacare, Bioiberica, Changzhou Qianhong Biopharma, Dr. Reddy’s Laboratories, Fresenius Kabi, Laboratorios Farmaceuticos ROVI, Leo Pharma, Nanjing King-Friend Biochemical Pharmaceutical, and Pfizer.
What are the upcoming trends in the heparin industry?
Key trends include the shift toward premium biosimilar and synthetic formulations, increasing adoption of low molecular weight heparins, and innovations in prefilled syringes, autoinjectors, and safety-engineered delivery systems to enhance patient compliance.
Which route of administration led the heparin market?
The subcutaneous segment led the market with a 57.6% share in 2024, driven by its convenience, reduced hospital visits, and suitability for long-term anticoagulant therapy.
What was the valuation of the porcine segment?
The porcine segment exhibited a significant CAGR of 7.3% in 2024, driven by its widespread use as a source for heparin production.
How much revenue did the low molecular weight heparin segment generate?
The low molecular weight heparin segment generated 79.6% of the market share in 2024 and is projected to exceed USD 8.6 billion by 2034, growing at a CAGR of 7.2% during the forecast period.
What is the market size of the heparin in 2024?
The market size was USD 5.4 billion in 2024, with a CAGR of 7.2% expected through 2034, driven by the increasing prevalence of cardiovascular and thrombotic disorders, rising surgical procedures, and expanding applications in dialysis, oncology, and medical devices.
What is the projected value of the heparin market by 2034?
The market is expected to reach USD 10.8 billion by 2034, driven by advancements in biosimilar and synthetic formulations, growing adoption of low molecular weight heparins, and innovations in delivery systems.
What is the projected size of the heparin market in 2025?
The market is expected to reach USD 5.8 billion in 2025.
Heparin Market Scope
Related Reports