Summary
Table of Content

Europe Gaucher Disease Drugs Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Europe Gaucher Disease Drugs Market Size
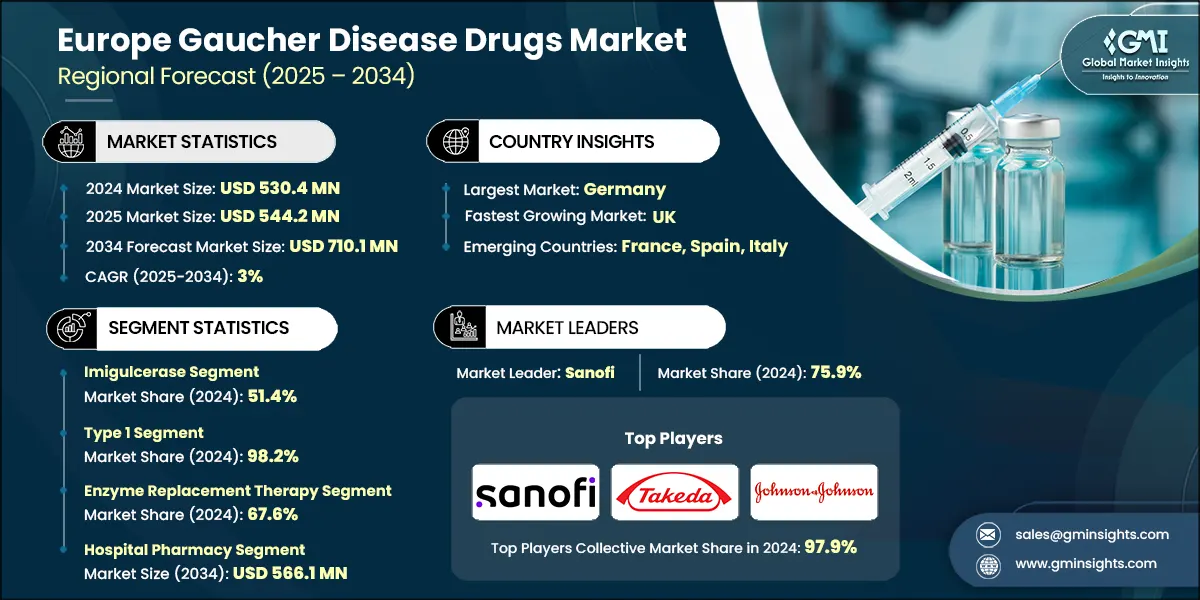
The Europe Gaucher disease drugs market was valued at USD 530.4 million in 2024. The market is expected to grow from USD 544.2 million in 2025 to USD 710.1 million in 2034, at a CAGR of 3% during the forecast period, according to the latest report published by Global Market Insights Inc.
To get key market trends
Europe Gaucher disease market is seeing steady growth driven by rising awareness about rare genetic disorders, enhanced diagnostic capacity, and the widening availability of enzyme replacement and substrate reduction therapy. Gaucher disease, a lysosomal storage disorder that results from deficiency of the enzyme glucocerebrosidase, has witnessed profound therapeutic developments in the last decade.
Europe Gaucher Disease Drugs Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 530.4 Million |
| Market Size in 2025 | USD 544.2 Million |
| Forecast Period 2025 - 2034 CAGR | 3% |
| Market Size in 2034 | USD 710.1 Million |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of Gaucher disease | Better tracking systems such as national registries are helping identify more cases, especially Type 1. This is increasing demand for long-term treatment options. |
| Growing investments for developing Gaucher disease therapies | European companies are investing in new treatments, supported by EU incentives. This is helping bring better and more advanced therapies to market. |
| Increasing awareness towards timely diagnosis and treatment | Doctors and patients are more informed, leading to faster diagnosis and treatment. This improves patient health and reduces complications. |
| Rising government support for rare disease therapies | EU policies and EMA support are helping companies develop and launch Gaucher drugs faster, making treatments more accessible across Europe. |
| Pitfalls & Challenges | Impact |
| High cost of therapies | Even with insurance and reimbursement systems, the cost of enzyme therapies is still a challenge for many healthcare systems. |
| Presence of stringent regulatory approval procedures | Rare disease drugs face tough regulatory steps and small patient pools, which can slow down development and increase costs. |
| Opportunities: | Impact |
| Emerging market expansion and local manufacturing | Reaching underserved areas in Europe and setting up local manufacturing can lower costs and improve access to treatment. |
| Substrate reduction therapy adoption | Oral SRTs offer a more convenient alternative to intravenous ERTs, improving patient compliance and expanding treatment options, especially in regions with limited infusion infrastructure. |
| Market Leaders (2024) | |
| Market Leaders |
75.9 % market share |
| Top Players |
Collective market share in 2024 is 97.9% |
| Competitive Edge |
|
| Regional Insights | |
| Largest market | Germany |
| Fastest growing market | UK |
| Emerging countries | France, Spain, Italy |
| Future outlook |
|
What are the growth opportunities in this market?
This market segment plays a pivotal role in transforming the treatment paradigm for lysosomal storage disorders, particularly Gaucher disease, by offering targeted therapies that address the underlying enzymatic deficiency. Leading companies in the Gaucher disease drug market including Sanofi, Takeda Pharmaceutical Company Limited, and Johnson & Johnson are at the forefront of innovation, driving therapeutic advancements and expanding global access. These players maintain their competitive edge through continuous R&D in next-generation therapies, strategic collaborations, and investments in rare disease platforms.
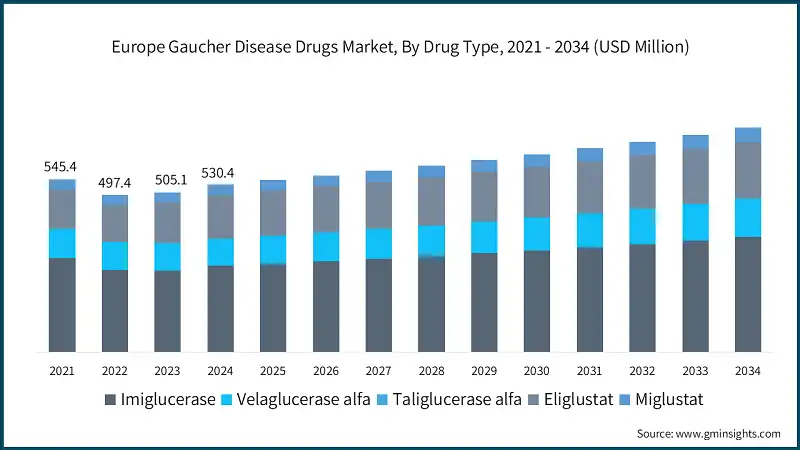
The Europe Gaucher disease drugs market is witnessing considerable growth, expanding from USD 545.4 million in 2021 to USD 505.1 million in 2023. The Gaucher disease drug market in Europe is experiencing steady growth, driven by enhanced rare disease infrastructure, national registries, and supportive regulatory frameworks. In France, the French Gaucher Disease Registry (FGDR) has documented 706 confirmed cases between 1980 and 2024, with 447 patients alive as of 2024. These figures underscore the importance of early diagnosis and sustained access to therapy across the region.
Moreover, Europe’s commitment to personalized medicine, equitable access, and collaborative research networks positions the region as a key contributor to global innovation in rare disease care. With strong regulatory frameworks and growing patient advocacy, the Gaucher disease market in Europe is poised for sustained therapeutic advancement.
Gaucher disease drugs help manage symptoms caused by a deficiency of the enzyme glucocerebrosidase. These medications either replace the missing enzyme or reduce the buildup of harmful substances in the body. Enzyme replacement therapies (ERT) such as imiglucerase, velaglucerase alfa, and taliglucerase alfa restore enzyme function. Substrate reduction therapies (SRT) such as eliglustat and miglustat lower the production of fatty substances that accumulate in organs. Doctors prescribe these drugs based on the type and severity of Gaucher disease. Treatment aims to improve quality of life and prevent complications.
Europe Gaucher Disease Drugs Market Trends
- The Gaucher disease drug market in Europe is expanding steadily, supported by a combination of medical advancements, regulatory incentives, and growing awareness. Gaucher disease, a rare genetic disorder caused by mutations in the GBA gene, is increasingly recognized as a priority within Europe’s rare disease framework. Improved diagnostic tools, wider adoption of newborn screening, and access to genetic counseling are enabling earlier detection and timely treatment, which is driving demand for effective therapies.
- Technological innovation is playing a key role in reshaping the treatment landscape. Europe is seeing increased use of digital diagnostics, AI-powered imaging tools, and gene therapy research, all of which are contributing to more personalized and predictive care. These advancements are helping clinicians tailor treatments to individual patient needs, particularly in complex cases such as neuronopathic Gaucher disease, where traditional enzyme therapies have limited effectiveness in addressing neurological symptoms.
- Research efforts in Europe are also exploring pluripotent stem cell platforms and advanced drug screening models to identify new compounds that could restore enzyme function. These approaches have potential applications not only in Gaucher disease but also in related conditions such as Parkinson’s disease.
- Additionally, the EU’s orphan drug designation (EU/3/20/2326) offers market exclusivity and regulatory support, making it easier for companies to bring high-cost therapies such as Enzyme Replacement Therapy (ERT) and Substrate Reduction Therapy (SRT) to market. These incentives are crucial for ensuring commercial viability and expanding patient access across Europe.
Europe Gaucher Disease Drugs Market Analysis
Learn more about the key segments shaping this market
Based on drug type, the Europe Gaucher disease drugs market is segmented into imiglucerase, velaglucerase alfa, taliglucerase alfa, eliglustat, and miglustat. The imiglucerase held a significant market share of 51.4% in 2024.
- Imiglucerase is a well-established enzyme replacement therapy used to treat Gaucher disease, a rare genetic condition caused by a deficiency in the enzyme glucocerebrosidase. By replacing the missing enzyme, Imiglucerase helps break down the accumulated glucocerebroside in cells, easing symptoms and improving patient health.
- Its strong clinical track record and long-term safety profile have made it a trusted choice among healthcare professionals across Europe. With approvals from major regulatory bodies and inclusion in treatment guidelines, Imiglucerase continues to be a preferred therapy for managing Gaucher disease.
- The therapy’s ease of use and reliable performance have contributed to its widespread adoption. Additionally, its availability through established distribution channels and patient support programs ensures consistent access and reinforces its leadership in the market.
- Thus, imiglucerase is expected to remain a cornerstone of Gaucher disease treatment in Europe, supported by ongoing demand, proven effectiveness, and its role in improving patient outcomes.
Based on disease type, the Europe Gaucher disease drugs market is segmented into type 1 and type 2. The type 1 segment held a significant market share of 98.2% in 2024.
- Type 1 Gaucher disease (GD1) is the most common and commercially important form of Gaucher disease in Europe. It presents systemic symptoms such as enlarged liver and spleen, blood-related issues, and bone complications but without neurological involvement. This makes GD1 more manageable and responsive to current therapies, which is why it remains the central focus for treatment development and commercialization.
- In Europe, awareness of GD1 is growing, especially among genetically predisposed populations. This has led to earlier diagnosis and consistent demand for treatment, creating a stable market segment that continues to attract investment from pharmaceutical companies.
- GD1 also benefits from clear clinical guidelines and supportive regulatory pathways across the region. These factors simplify market entry, support reimbursement processes, and give companies greater confidence in long-term planning. As a result, GD1 continues to be a strategic priority in Europe’s rare disease landscape offering both strong clinical relevance and solid commercial potential.
Based on the therapy type, the Europe Gaucher disease drugs market is segmented into enzyme replacement therapy, and substrate replacement therapy. The enzyme replacement therapy segment held a significant market share of 67.6% in 2024.
- Enzyme replacement therapy (ERT) continues to be a foundational treatment for Gaucher disease in Europe, especially for patients with Type 1. This approach involves administering synthetic glucocerebrosidase to replace the deficient enzyme, helping reduce the buildup of harmful substances in organs such as the liver, spleen, and bone marrow.
- ERT has earned the confidence of clinicians and regulators alike, due to its consistent effectiveness and strong safety record. Therapies such as imiglucerase, velaglucerase alfa, and taliglucerase alfa are approved by European health authorities and are widely recommended in clinical guidelines. Their long-standing use has built trust among healthcare providers and patients, reinforcing ERT’s role as a reliable treatment option.
- In Europe, the availability of ERT through established healthcare systems and patient support programs has made routine disease management more accessible. This has enabled earlier intervention and better long-term health outcomes for many individuals living with Gaucher disease.
- Hence, as healthcare access continues to improve across the region, demand for ERT is expected to grow. This is driving further investment in manufacturing capacity, distribution networks, and patient services, ensuring that ERT remains a key driver of market growth and a cornerstone of care in the European Gaucher disease landscape.
Learn more about the key segments shaping this market
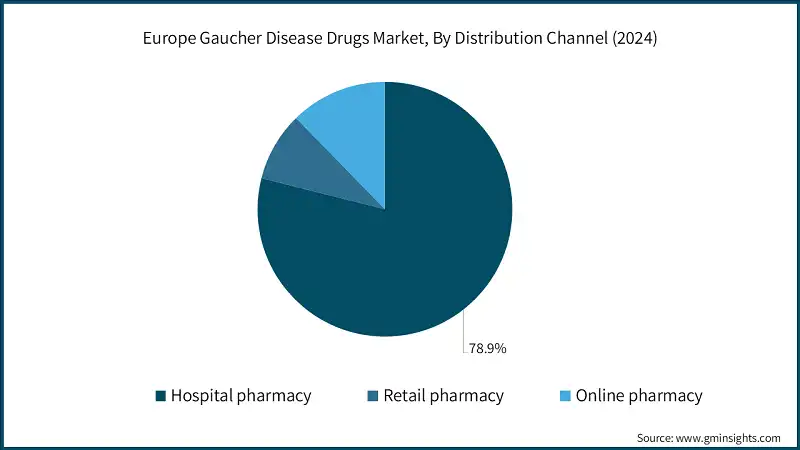
Based on distribution channel, the Europe Gaucher disease drugs market is categorized into hospital pharmacy, retail pharmacy, and online pharmacy. Among these, the hospital pharmacy segment accounts for 78.9% of the total market share. The growth is projected to continue, with the segment expected to reach USD 566.1 million by 2034.
- Hospital pharmacies are increasingly integral to the delivery of Gaucher disease therapies, particularly enzyme replacement treatments that require specialized handling and administration. Their infrastructure supports safe infusion practices, ensuring patients receive timely and accurate dosing under clinical supervision.
- The expansion of hospital pharmacy services in Europe is enhancing treatment continuity and patient adherence. With access to trained staff and monitoring systems, hospitals can manage complex regimens more effectively, reducing therapy interruptions and improving long-term outcomes for Gaucher disease patients.
- Hospitals also serve as key entry points for diagnosis and care coordination. Their ability to integrate lab testing, specialist consultations, and pharmacy services streamlines the patient journey from identification to treatment initiation, accelerating therapeutic impact and improving efficiency.
- Therefore, as awareness of Gaucher disease grows, hospitals are increasingly equipped to support rare disease therapies. This trend is improving geographic access, particularly in emerging markets, and enabling more equitable distribution of advanced treatments to patients who rely on institutional care.
Looking for region specific data?
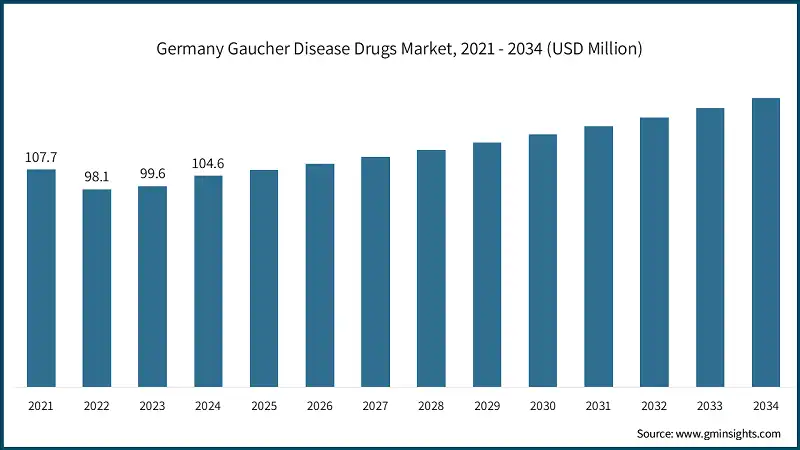
Germany dominates the Europe Gaucher disease drugs market, showcasing strong growth potential.
- Germany is a strategically valuable part of the European Gaucher disease pharmaceutical market, with its well-developed healthcare infrastructure, robust public funding for rare disease management, and increasing focus on precision medicine. The well-developed diagnostic capability, with a high prevalence of access to enzyme assays and genetic testing, allows for early detection and successful management of Gaucher disease, especially Type 1.
- The availability of approved enzyme replacement therapies such as imiglucerase and velaglucerase alfa, along with increasing adoption of oral substrate reduction therapies such as eliglustat, has significantly improved treatment accessibility and patient outcomes. Germany’s leadership in pharmacogenomics further enhances personalized care, with CYP2D6 genotyping increasingly used to guide individualized dosing strategies and optimize therapeutic efficacy.
- In addition, Germany is leading the way in bringing digital health solutions and remote monitoring technologies into chronic disease management. These technologies enable real-time monitoring of treatment, enhance compliance, and complement the country's overall movement toward value-based care. An active regulatory framework, coupled with an emphasis on clinical data, safety, and interoperability, enables quick uptake of new therapies and enables market growth sustainably.
UK Gaucher disease drugs market is anticipated to grow at a significant CAGR over the analysis period.
- The UK represents a vital segment of the European Gaucher disease drug market, supported by its strong healthcare infrastructure, national rare disease strategy, and growing focus on personalized medicine. The country’s commitment to early diagnosis and coordinated care is reflected in its Rare Diseases Framework and England Rare Diseases Action Plan, which prioritize faster diagnosis, improved access to specialist treatments, and better integration of genomic data into clinical practice.
- Specialist treatment centers across the UK offer comprehensive care for Gaucher patients, including enzyme assays, genetic testing, and access to both Enzyme Replacement Therapy (ERT) and Substrate Reduction Therapy (SRT). Therapies such as imiglucerase, velaglucerase alfa, and eliglustat are widely available helping streamline treatment and improve patient convenience.
- Moreover, digital health tools and remote monitoring are being integrated into chronic disease management, helping track treatment adherence and outcomes in real time. These innovations are supported by the UK’s emphasis on value-based care, data interoperability, and collaborative research across public and private sectors.
- With a robust regulatory environment, active patient advocacy, and continued investment in rare disease research, the UK is well-positioned to drive innovation and growth in the Gaucher disease drug market.
France Gaucher disease drugs market is anticipated to grow at a significant CAGR over the analysis period.
- France plays a pivotal role in the European Gaucher disease drug market, backed by a mature healthcare system, strong public investment in rare disease research, and a national commitment to personalized medicine. The country’s rare disease strategy is anchored by the French Gaucher Disease Registry (FGDR), which has tracked 706 confirmed cases between 1980 and 2024, with 447 patients still alive.
- The country is also advancing in precision medicine, supported by initiatives such as Genomic Medicine France 2025 and the Fourth National Rare Diseases Plan (PNMR4). These programs promote genome sequencing, digital health integration, and early screening, helping tailor therapies to individual genetic profiles and improve outcomes.
- With a robust regulatory framework, coordinated national research efforts, and a strong focus on patient-centric care, France is well-positioned to drive continued growth and innovation in the Gaucher disease drug market.
Spain Gaucher disease drugs market is anticipated to grow at a significant CAGR over the analysis period.
- Spain is emerging as a strategically important market for Gaucher disease therapies in Europe, supported by a growing rare disease infrastructure, national registries, and strong public health initiatives. The Spanish Registry of Gaucher Disease (REsEG), established in 1993, has played a key role in tracking patient data across the country.
- Spain offers access to key Enzyme Replacement Therapies (ERTs) such as imiglucerase and velaglucerase alfa and is seeing rising adoption of oral Substrate Reduction Therapies (SRTs) such as eliglustat and miglustat. These therapies are available through specialized treatment centers helping improve patient outcomes and treatment adherence.
- With continued investment in research, regulatory support for orphan drugs, and strong collaboration between public institutions and industry, Spain is well-positioned to contribute meaningfully to the growth and innovation of the Gaucher disease drug market in Europe.
Italy Gaucher disease drugs market is anticipated to grow at a significant CAGR over the analysis period.
- Italy is a growing and strategically important market for Gaucher disease therapies in Europe, supported by a robust national rare disease framework, expanding diagnostic capabilities, and increasing adoption of personalized care models. The country’s healthcare system has made rare diseases a public health priority, with the National Rare Disease Plan 2023–2026 and laws such as L.175/2021 providing a structured approach to diagnosis, treatment access, and orphan drug development.
- Italy has a well-established network of accredited rare disease centers and a national registry hosted by the Istituto Superiore di Sanità (ISS). These resources support early diagnosis and care for Gaucher disease patients, particularly those with Type 1.
- Italy is also advancing with initiatives such as MetabERN. These innovations are helping improve treatment adherence, enable real-time tracking, and support the country’s shift toward value-based care.
- With strong regulatory support, active public-private collaboration, and a growing focus on personalized and digital care, Italy is well-positioned to drive innovation and growth in the European Gaucher disease drug market.
Europe Gaucher Disease Drugs Market Share
The Gaucher disease drug market in Europe is shaped by a mix of established pharmaceutical leaders and emerging innovators, creating a dynamic and competitive environment. Sanofi, Takeda Pharmaceutical Company Limited, and Johnson & Johnson are the leading players in the region, holding 97.9% market share through their proven treatment portfolios, strong regulatory presence, and continued investment in rare disease innovation.
These companies have built solid positions in Europe by combining regulatory expertise, strategic partnerships with academic and clinical institutions, and expansion into underserved areas. Their flagship enzyme replacement therapies such as Cerezyme, VPRIV, and Zavesca are widely adopted across European healthcare systems, supported by long-term safety data and inclusion in national treatment guidelines.
In addition to their established therapies, these companies are actively investing in next-generation solutions, including oral substrate reduction therapies and gene therapy candidates, aimed at improving patient outcomes and addressing unmet needs in both Type 1 and neuronopathic Gaucher disease. Thus, the European Gaucher disease drug market is becoming increasingly competitive, with deeper therapeutic diversification and a growing emphasis on personalized care. As players in the market continue to innovate and expand their offerings, the market is expected to evolve toward more accessible, effective, and patient-focused treatment solutions.
Europe Gaucher Disease Drugs Market Companies
Prominent players operating in the Europe Gaucher disease drugs industry are as mentioned below:
- Dipharma SA
- Generium
- ISU ABXIS
- Johnson & Johnson
- Pfizer Inc.
- Prevail Therapeutics
- Sanofi
- Takeda Pharmaceutical Company Limited
- Sanofi
Sanofi is a leading player in the European Gaucher disease drug market, best known for its flagship therapy Cerezyme (imiglucerase) a recombinant enzyme replacement treatment approved for both adult and pediatric patients (aged 2 and above) with Type 1 Gaucher disease. With decades of clinical experience, Sanofi has positioned Cerezyme as a benchmark therapy across Europe, supported by strong safety data, widespread regulatory approvals, and broad clinical adoption.
Takeda Pharmaceutical Company Limited is a key player in the European Gaucher disease drug market, known for its enzyme replacement therapy VPRIV (velaglucerase alfa). This treatment is approved for long-term management of Type 1 Gaucher disease and is widely available across European healthcare systems. Backed by strong clinical data and regulatory approvals within the EU. Takeda’s commitment to rare disease care is reflected in its investment in research, patient support programs, and its ability to deliver therapies through a well-established distribution network across Europe.
Johnson & Johnson is a notable player in the Gaucher disease drug market, offering ZAVESCA (miglustat) an oral therapy indicated for adults with mild to moderate Type 1 Gaucher disease who are not suitable candidates for enzyme replacement therapy (ERT). Johnson & Johnson’s focus on oral therapeutics and niche patient populations reinforces its role in diversifying the Gaucher treatment landscape.
Europe Gaucher Disease Drugs Industry News
- In April 2022, Sanofi initiated a Phase 3 clinical trial to assess the efficacy and safety of venglustat, an oral substrate reduction therapy, versus Cerezyme (imiglucerase) in patients with Gaucher disease type 3 (GD3). The randomized, double-blind study is aimed at targeting neurological improvement and systemic stability over 52 weeks, followed by an open-label extension. The trial enrolled 43 participants and is expected to complete primary analysis by September 2025, with full study completion projected for October 2026. This trial represented a significant step in advancing oral treatment options for Gaucher disease type 3, with the potential to reduce treatment burden and improve patient quality of life.
The Europe Gaucher disease drugs research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD million and volume in Units from 2021 - 2034 for the following segments:
Market, By Drug Type
- Imiglucerase
- Velaglucerase alfa
- Taliglucerase alfa
- Eliglustat
- Miglustat
Market, By Disease Type
- Type 1
- Type 3
Market, By Therapy Type
- Enzyme replacement therapy
- Substrate replacement therapy
Market, By Distribution Channel
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
The above information is provided for the following countries:
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
Frequently Asked Question(FAQ) :
Who are the key players in the Europe Gaucher disease drugs market?
Key players include Dipharma SA, Generium, ISU ABXIS, Johnson & Johnson, Pfizer Inc., Prevail Therapeutics, Sanofi, and Takeda Pharmaceutical Company Limited.
Which distribution channel led the Europe Gaucher disease drugs market in 2024?
Hospital pharmacies dominated the market with a 78.9% share in 2024 and are projected to reach USD 566.1 million by 2034.
What are the upcoming trends in the Europe Gaucher disease drugs industry?
Key trends include the adoption of digital diagnostics, AI-driven imaging tools, gene therapy research, and personalized treatment approaches for complex cases like neuronopathic Gaucher disease.
What was the valuation of the enzyme replacement therapy segment in 2024?
The enzyme replacement therapy segment held a significant market share of 67.6% in 2024.
What was the market share of imiglucerase in 2024?
Imiglucerase held a significant market share of 51.4% in 2024, leading the drug type segment.
What was the market share of type 1 Gaucher disease drugs in 2024?
The type 1 segment dominated with a market share of 98.2% in 2024.
What is the projected size of the Europe Gaucher disease drugs market in 2025?
The market is expected to reach USD 544.2 million in 2025.
What is the projected value of the Europe Gaucher disease drugs market by 2034?
The market is expected to reach USD 710.1 million by 2034, supported by technological innovations such as AI-powered imaging tools and gene therapy research.
What was the market size of the Europe Gaucher disease drugs in 2024?
The market size was USD 530.4 million in 2024, with a CAGR of 3% expected through 2034, driven by advancements in diagnostics, regulatory incentives, and growing awareness of rare diseases.
Europe Gaucher Disease Drugs Market Scope
Related Reports


