Companion Diagnostics Market worth over $6.5bn by 2025
Published Date: July 2019
Companion Diagnostics Market size is set to surpass USD 6.5 billion by 2025, according to a new research report by Global Market Insights Inc.
Growing adoption of drug diagnostics co-development model to identify individual’s response to treatment options will augment the market revenue. Companion diagnostics are being developed along with the drug and used to check its effectiveness as well as associated adverse reactions. Moreover, complementary diagnostic tests enable monitoring of drug response over the entire treatment course. They further provide access to person’s genetic, proteomic and metabolomics data resulting in better outcomes.
Improving regulatory scenario that facilitates entry of products will render a significant positive impact on the companion diagnostics industry size. Innovative products are being developed by players in the market to identify appropriate drug diagnostic combination. Regulatory bodies ensure that effective and safe drugs are available across the globe.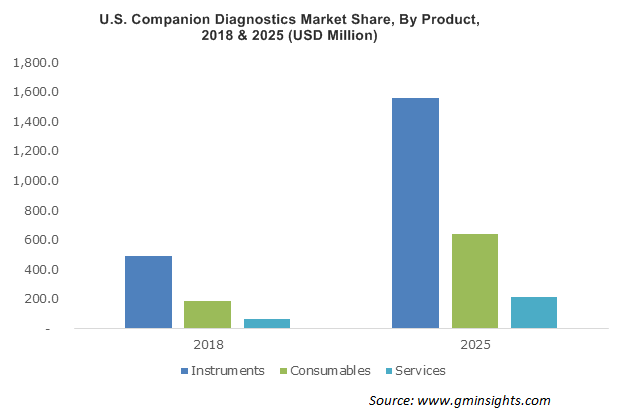
Get more details on this report - Request Free Sample PDF
Instruments segment revenue was more than USD 1.2 billion in 2018. Emphasis on R&D activities undertaken by industry players for development of innovative instruments that assist in accurate diagnosis will drive the segment size. Technologically advanced instruments require less amount of media and reagents and delivers results in precise time. These instruments are used for multiple companion diagnostics test such as non-small cell lung cancer, melanoma, breast, colorectal and ovarian cancers.
Breast cancer application segment is anticipated to witness around 19% growth till 2025. Increasing prevalence of breast cancers will boost demand for companion diagnostics for effective and accurate treatment. According to CDC, in 2016, 245,299 incidences of breast cancer were reported in the U.S. alone. Large patient pool will augment demand for companion diagnostics as standard drugs are not always effective in all patients. HER2 protein is associated with breast cancer and often used as biomarker.
Browse key industry insights spread across 110 pages with 101 market data tables & 9 figures & charts from the report, “Companion Diagnostics Market Size By Product (Instruments, Consumables, Services), By Disease Indication (Breast cancer, Lung cancer, Colorectal cancer, Skin cancer), By Technology (Immunohistochemistry, In Situ Hybridization, Polymerase Chain Reaction, Genetic Sequencing), By End-use(Hospitals, Diagnostic Laboratories), Industry Analysis Report, Regional Outlook, Application Potential, Competitive Market Share & Forecast, 2019 - 2025” in detail along with the table of contents: https://www.gminsights.com/industry-analysis/companion-diagnostics-market
Immunohistochemistry segment size was over USD 500 million in 2018. Immunohistochemistry involves identification of antigens through antigen antibody binding principle. This technology further enables evaluation of drug contingency with significant accuracy. Effective results obtained with the usage of immunohistochemistry technology will augment its preference.
Diagnostic laboratories end-user segment is estimated to expand at 19.5% CAGR during the forecast timeframe. Increasing government funding and growing awareness pertaining to advantages of early diagnosis will escalate number of diagnostics procedures. Additionally, these laboratories provide disease specific diagnostics for several types of cancers, that will prove beneficial for the segment growth. Moreover, diagnostics laboratories incorporate technologically superior devices providing efficient results.
North America companion diagnostics market value was around USD 800 million in 2018 owing to high disposable income and availability of developed healthcare infrastructure. Robust research activities aimed at development of targeted drugs and therapies will augment the market demand in the region. Demographic trends such as rising geriatric population susceptible to chronic disorders and consequent increase in disease prevalence will support the industry growth.
Notable players operational in the market are Roche, Pfizer, Merck, Astra Zeneca, Bristol Myers Squibb, Amgen, Biogen, Eli Lilly, Myriad Genetics, Johnson & Johnson, Becton, Dickinson and Company and Abbott. The market players are implementing strategic initiatives such as geographic expansions, product launches, collaborations, acquisitions, and mergers to acquire potential market share.





