Summary
Table of Content

U.S. Peripheral Intravenous Catheters Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
U.S. Peripheral Intravenous Catheters Market Size
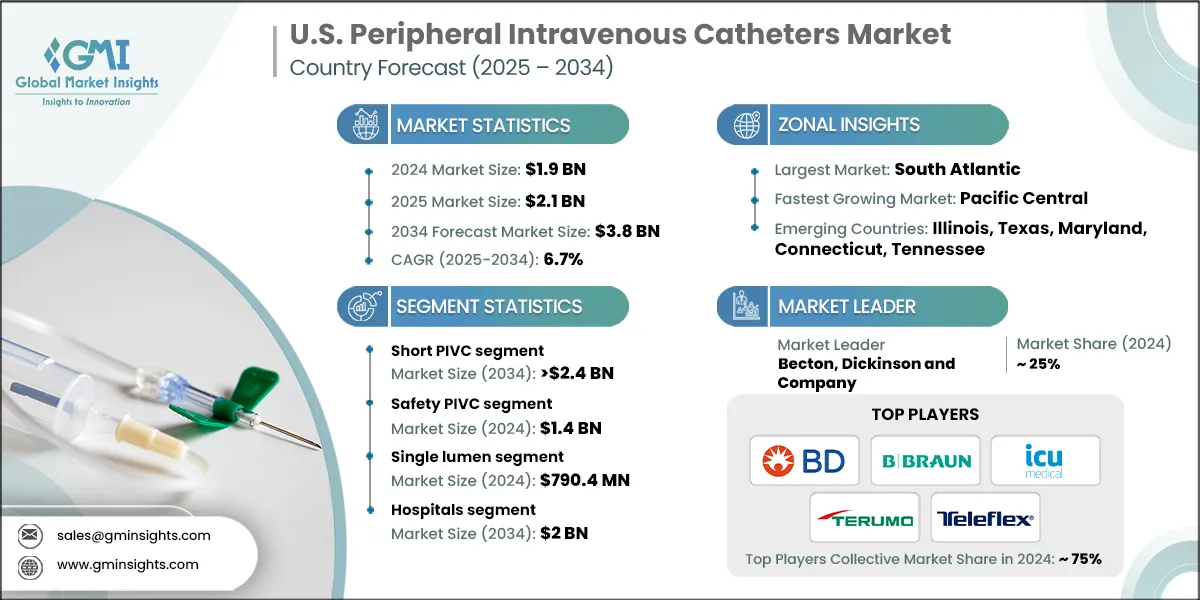
The U.S. peripheral intravenous catheters market was valued at USD 1.9 billion in 2024. The market is expected to reach from USD 2.1 billion in 2025 to USD 3.8 billion in 2034, growing at a CAGR of 6.7% during the forecast period, according to the latest report published by Global Market Insights Inc. The high market growth is attributed to the rising burden of chronic and lifestyle diseases, increasing number of hospitalizations and emergency care procedures, expansion of outpatient and home infusion therapy, and advancements in catheter design and safety features, among other contributing factors.
To get key market trends
Peripheral intravenous catheters (PIVCs) are small, flexible tubes inserted into peripheral veins, usually in the hand or arm, to deliver medications, fluids, or nutrients directly into the bloodstream. They provide short-term venous access in hospitals, emergency care, and outpatient settings. PIVCs are commonly used for treatments such as hydration, antibiotic therapy, pain management, and blood sampling.
The major contributors to the U.S. peripheral intravenous catheters market are Becton, Dickinson and Company, B. BRAUN, ICU Medical, TERUMO, and Teleflex. These companies strengthen their competitive position by ongoing product originality, worldwide market presence, and huge allocations in the research and development area.
U.S. Peripheral Intravenous Catheters Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 1.9 Billion |
| Market Size in 2025 | USD 2.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 6.7% |
| Market Size in 2034 | USD 3.8 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising incidence of chronic diseases and cancer | The growing number of patients with chronic illnesses and cancer has increased demand for frequent IV therapies, driving PIVC utilization in hospitals and outpatient settings. |
| Increasing hospital admissions and outpatient procedures | Higher surgical volumes and procedural care have expanded the need for short-term venous access, supporting market growth. |
| Preference for peripheral IVs over central lines for short-term access | Clinicians increasingly favor PIVCs for safer, less invasive, and cost-effective short-term treatments, boosting adoption. |
| Technological improvements in catheter materials and designs | Innovations like anti-microbial coatings, flexible materials, and safety-engineered designs have enhanced patient outcomes, encouraging wider use. |
| Pitfalls & Challenges | Impact |
| Risk of catheter-related bloodstream infections (CRBSIs) and complications | Infection risks remain a significant concern, limiting prolonged use and necessitating stringent monitoring, which can restrain market expansion. |
| Strict regulatory requirements and lengthy approvals | Compliance with FDA standards and approval timelines slows product launches and increases development costs, affecting market pace. |
| Opportunities: | Impact |
| Development of antimicrobial/coated and biofilm-resistant catheters | Emerging catheter technologies with infection-prevention features are expected to reduce CRBSIs, enhance patient safety, and drive higher adoption. |
| Market Leaders (2024) | |
| Market Leaders |
~ 25% Market share |
| Top Players |
Collective market share in 2024 is ~ 75% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | South Atlantic |
| Fastest growing market | Pacific Central |
| Emerging states | Illinois, Texas, Maryland, Connecticut, Tennessee |
| Future outlook |
|
What are the growth opportunities in this market?
The market has increased from USD 1.5 billion in 2021 and reached USD 1.8 billion in 2023, with the historic growth rate of 8.5%. The growth is attributed to the rising prevalence of chronic diseases requiring frequent intravenous therapies, increasing surgical and hospitalization rates, and advancements in catheter materials and safety features that enhance patient comfort and reduce infection risks.
Moreover, the increasing incidence of cardiovascular diseases, cancer, diabetes, and renal disorders in the U.S. drives higher hospital admissions and extended treatments requiring intravenous (IV) therapy. For instance, according to the Centers for Disease Control and Prevention (CDC), in 2022, cardiovascular diseases affected 18.2 million adults, while the American Cancer Society reported 1.9 million new cancer cases in 2023.
The National Diabetes Statistics Report 2023 indicated that 37.3 million Americans have diabetes. Peripheral IV catheters play a vital role in drug administration, hydration, and chemotherapy. The aging population's susceptibility to chronic diseases increases the reliance on peripheral IV catheters, with the U.S. Census Bureau projecting that adults aged 65 and older will reach 77 million by 2024, supporting continuous market expansion.
Furthermore, technological developments have introduced needle-stick injury prevention systems, closed IV catheter systems, and antimicrobial-coated catheters. These innovations improve insertion success, patient comfort, and infection control outcomes. Hospitals increasingly adopt safety-engineered catheters to reduce catheter-related bloodstream infections (CRBSIs) and meet nursing safety protocols. Enhanced device reliability drives product replacement demand as well.
U.S. Peripheral Intravenous Catheters Market Trends
The market is experiencing several notable trends that are shaping its growth and development. Factors such as the growth of home and ambulatory IV therapy, the shift toward safety-engineered and antimicrobial catheters, the integration of smart and technology-enhanced devices, and the increased focus on minimally invasive and patient-friendly designs, among others, are collectively driving industry growth.
- Hospitals and clinics in the U.S. are increasingly adopting safety-engineered catheters to minimize needle-stick injuries and reduce infection risks. Antimicrobial-coated PIV catheters are gaining popularity to prevent catheter-related bloodstream infections (CRBSIs). This trend reflects a stronger emphasis on patient safety and regulatory compliance. As a result, providers are willing to invest in advanced, higher-cost devices.
- The expansion of outpatient care and home infusion therapies is driving demand for portable and easy-to-use PIV catheters. Patients with chronic illnesses, dehydration, or long-term antibiotic needs increasingly receive care outside hospital settings. Catheter designs that enable safe self-administration or home nursing use are trending. This shift reduces hospital stays while maintaining continuous IV therapy.
- Moreover, these catheters are increasingly being tailored for specific applications such as chemotherapy, long-term antibiotic therapy, and critical care. Specialty catheters with enhanced tip designs, antimicrobial coatings, and securement systems are being widely adopted. Clinicians are favouring products that improve therapy efficiency and reduce complications. This trend is driving niche growth segments within the broader PIV catheter market.
U.S. Peripheral Intravenous Catheters Market Analysis
Learn more about the key segments shaping this market
Based on the product, the U.S. peripheral intravenous catheters market is segmented into short PIVC and integrated/closed PIVC. The short PIVC segment has asserted its dominance in the market by securing a significant market share of 63.9% in 2024 owing to its widespread use in hospitals and outpatient settings, ease of insertion, cost-effectiveness, and suitability for short-to medium-term intravenous therapies across diverse patient populations. The segment is expected to exceed USD 2.4 billion by 2034, growing at a CAGR of 6.5% during the forecast period.
On the other hand, the integrated/closed PIVC segment is expected to grow with a CAGR of 7%. The growth of this segment is driven by increasing demand for safety-engineered, needle-free systems that reduce contamination and needlestick injuries, along with regulatory emphasis on healthcare worker and patient safety.
- The short PIVC segment continues to dominate the market. Short PIVCs are extensively employed in emergency rooms, intensive care units, and surgical wards due to their quick insertion and immediate use. They are the means of first choice to provide rapid vascular access for fluid resuscitation, medication administration, and blood sampling. The increasing number of hospitalizations and emergency visits in the U.S. drives demand. Their ease of use makes them the preferred choice in time-sensitive clinical situations.
- Conditions such as cardiovascular disorders, diabetes, cancer, and infections often require repeated IV therapy. Short PIVCs are commonly employed for short-term treatments, hydration, and drug delivery. As chronic and acute illnesses continue to grow in prevalence, healthcare providers increasingly rely on these catheters for efficient patient management. This factor supports sustained market growth.
- The integrated/closed PIVC segment held a revenue of USD 700.7 million in 2024, with projections indicating a steady expansion at 7% CAGR from 2025 to 2034. Rising focus on healthcare worker safety and infection control is driving adoption of integrated, needle-free catheter systems. Additionally, regulatory guidelines and hospital safety protocols encourage the use of closed PIVCs to minimize contamination and needlestick risks.
Based on technology, the U.S. peripheral intravenous catheters market is segmented into safety PIVC and conventional PIVC. The safety PIVC segment dominated the market in 2024, accounting for USD 1.4 billion and is anticipated to grow at a CAGR of 6.8% during the forecast period.
- Safety PIVCs are designed with engineered protection mechanisms to reduce needle-stick injuries among healthcare professionals. With the U.S. Occupational Safety and Health Administration (OSHA) and other regulatory guidelines emphasizing staff safety, hospitals increasingly adopt these catheters. This trend minimizes occupational hazards and associated treatment costs, driving higher adoption rates.
- Moreover, healthcare providers are increasingly aware of occupational and patient safety risks associated with traditional IV catheters. Federal regulations, such as the Needlestick Safety and Prevention Act, encourage the use of safer devices. Hospitals and outpatient centers adopt safety PIVCs to comply with legal requirements and improve safety metrics, boosting market demand.
- The conventional PIVC segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 6.3% over the forecast period. Conventional PIVCs remain widely used due to their affordability and simplicity, making them suitable for routine intravenous therapies. Their broad availability and ease of use support adoption in both hospital and outpatient settings, especially in cost-sensitive environments.
Based on lumen, the U.S. peripheral intravenous catheters market is segmented into single lumen, double lumen, and multi-lumen. The single lumen segment dominated the market in 2024, accounting for USD 790.4 million and is anticipated to grow at a CAGR of 6.5% during the forecast period.
- Single lumen catheters are generally less expensive than multi-lumen ones, thus being economically attractive for numerous uses. They are appropriate for patients without the need for multiple simultaneous infusions, which constitute the majority of general wards. The price of such devices does not limit their use in hospitals, ambulatory care, and home healthcare services.
- Further, these are less complicated to insert and operate, thus resulting in a higher rate of success on the first attempt and less discomfort for the patient. Nurses and clinicians consider them handy for quick procedures, especially in pediatric, geriatric, and outpatient settings. Their simplicity contributes to clinical efficiency and patient satisfaction.
- The double lumen segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 7.1% over the forecast period. Double-lumen PIVCs enable simultaneous administration of multiple fluids or medications, reducing the need for repeated venipunctures. They are preferred in intensive care and complex treatment settings where efficient and continuous intravenous therapy is required.
- The multi-lumen segment accounted for significant revenue in 2024 and is anticipated to grow at a CAGR of 6.2% over the forecast period. Multi-lumen PIVCs allow the delivery of several medications or fluids concurrently, supporting complex treatment protocols in critical care. They enhance workflow efficiency and reduce vascular access complications in patients requiring intensive or long-term intravenous therapy.
Learn more about the key segments shaping this market
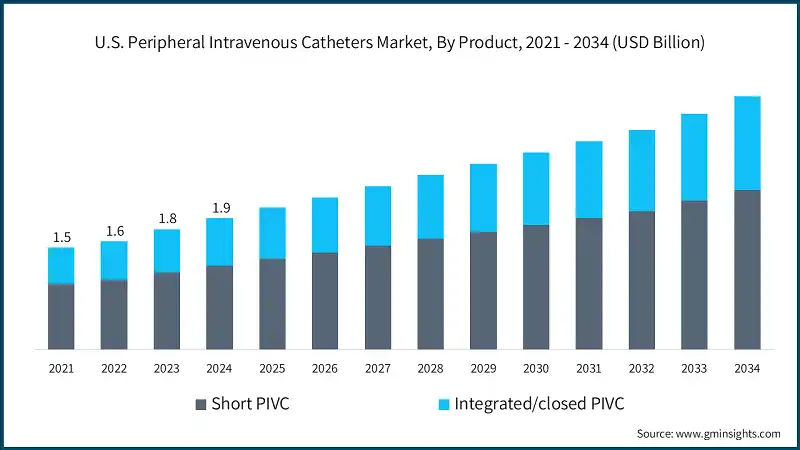
Based on end use, the U.S. peripheral intravenous catheters market is segmented into hospitals, ambulatory surgical centers, and other end users. The hospitals segment dominated the market with a revenue share of 54.3% in 2024 and is expected to reach USD 2 billion within the forecast period.
- Hospitals in the U.S. manage a large number of patients requiring intravenous therapy for surgeries, chronic diseases, trauma, and acute illnesses. The need for rapid vascular access in emergency, ICU, and general wards drives substantial PIVC usage. High patient turnover ensures continuous and recurring demand for catheters, supporting steady market growth.
- Additionally, hospitals prioritize safety, efficiency, and infection control, leading to the adoption of advanced PIVCs such as safety catheters, antimicrobial-coated devices, and closed systems. These technologies reduce catheter-related bloodstream infections (CRBSIs) and needle-stick injuries. Hospitals’ willingness to invest in premium products boosts demand for technologically enhanced PIVCs.
- The ambulatory surgical centers segment held a revenue of USD 523.3 million in 2024, with projections indicating a steady expansion at 7.4% CAGR from 2025 to 2034. Ambulatory surgical centers increasingly adopt PIVCs for short-duration procedures, anaesthesia administration, and postoperative fluid therapy. Their preference for cost-effective, easy-to-use catheters supports efficient patient throughput and reduced procedural times.
Looking for region specific data?
South Atlantic Peripheral Intravenous Catheters Market
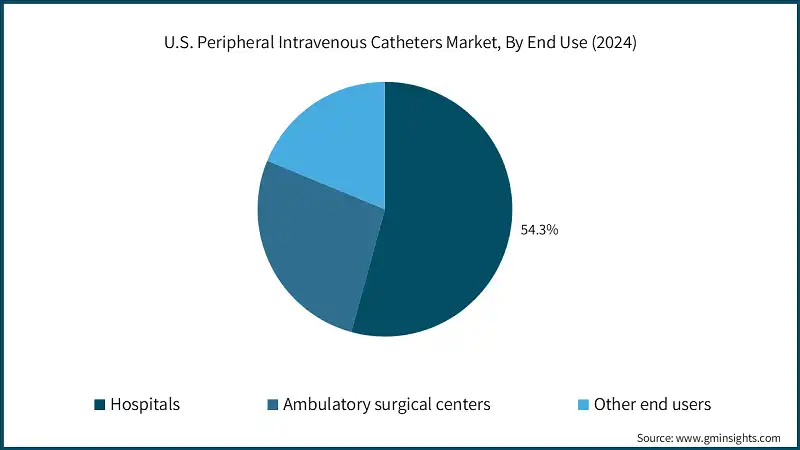
The South Atlantic zone dominated the U.S. peripheral intravenous catheters market with the leading market share of 19.4% in 2024.
- The South Atlantic region, including states like Florida, Georgia, and North Carolina, has a very dense hospital network along with specialty clinics and outpatient centers. Due to this high concentration, the demand for PIVCs has increased to meet the needs of routine IV therapy, emergency care, and surgical procedures. Both urban and suburban areas with large healthcare infrastructures are driving consistent catheter consumption.
- The region has a significant elderly population, especially in states like Florida, which results in higher cases of chronic diseases such as cardiovascular disorders, diabetes, and cancer. Such patients will require IV therapy frequently for hydration, medications, and chemotherapy. The aging demographic is a source that ensures continuous demand for PIVCs in hospital and outpatient settings.
- In addition, home infusion therapy and outpatient care are growing rapidly in the South Atlantic states due to cost containment and patient convenience trends. Peripheral IV catheters are essential for these services, providing safe and easy vascular access outside hospital settings. This shift from inpatient to outpatient and home care contributes to regional market growth.
Pacific Central Peripheral Intravenous Catheters Market
The Pacific Central peripheral intravenous catheters market accounted for USD 252.9 million in 2024 and is anticipated to show lucrative growth over the forecast period.
- Pacific Central states like California and Nevada have highly advanced hospitals, research centers, and specialty clinics. These top-notch facilities require high-quality PIVCs to carry out complex procedures, provide intensive care, and manage a large number of patients. The concentration of highly technologically advanced healthcare institutions is the main reason for the use of premium and safety-enhanced catheters.
- Many hospitals in the Pacific Central region are affiliated with academic institutions and clinical research programs. This encourages the use of innovative IV catheter technologies, such as smart PIVCs with monitoring capabilities. Research-driven healthcare centers adopt new devices faster, increasing regional market penetration and demand.
Northeast Peripheral Intravenous Catheters Market
The Northeast peripheral intravenous catheters market is anticipated to grow at the highest CAGR of 7.8% during the analysis timeframe.
- The Northeast, including states like New York, Massachusetts, and Pennsylvania, has a high density of hospitals, specialty centers, and academic medical institutions. This concentration ensures consistent demand for PIVCs across diverse care settings, from emergency care to oncology and cardiology wards. The region’s advanced healthcare infrastructure supports high catheter utilization.
- The Northeast is home to numerous top-ranked academic medical centers and research hospitals, which drive the adoption of advanced and experimental IV therapies. Safety-engineered, antimicrobial, and smart PIVCs are more rapidly introduced in these institutions. This research-focused environment fuels innovation adoption and increases market demand.
U.S. Peripheral Intravenous Catheters Market Share
The market is highly competitive, driven by innovation, safety features, and hospital procurement preferences. Companies focus on developing advanced safety-engineered catheters, needle-free connectors, and smart PIVCs to enhance patient safety and procedural efficiency.
Leading industry players such as Becton, Dickinson and Company, B. BRAUN, ICU Medical, TERUMO, and Teleflex hold around 75% of the market share in the competitive U.S. landscape, reflecting strong brand recognition, extensive distribution networks, and trusted clinical performance.
In addition to product innovation, companies compete through strategic partnerships with hospitals, outpatient centers, and home healthcare providers. Emphasis on regulatory compliance, cost-effectiveness, and training for healthcare professionals further strengthens market positioning. Smaller and regional players target niche applications or cost-sensitive segments, providing tailored solutions to outpatient clinics and ambulatory surgical centers.
Continuous R&D investment, focus on infection control, and adoption of digital health-enabled catheters are expected to shape competitive dynamics and maintain high entry barriers for new entrants.
U.S. Peripheral Intravenous Catheters Market Companies
A few of the prominent players operating in the U.S. peripheral intravenous catheters industry include:
- Access Vascular
- AngioDynamics
- B. BRAUN
- Becton, Dickinson and Company
- DELTA MED
- ICU Medical
- Lineus Medical
- MEDEREN
- MedSource Labs
- Retractable Technologies
- Teleflex
- TERUMO
- VYGON
- Becton, Dickinson and Company (BD)
BD is a market leader in safety-engineered PIVCs, offering products like Nexiva and Insyte Autoguard that reduce needlestick injuries and improve patient safety. Its strong R&D capabilities and global distribution network ensure high adoption across hospitals, outpatient centers, and home healthcare.
B. Braun’s PIVCs, such as the Introcan Safety series, emphasize passive safety mechanisms and ergonomic design to minimize occupational hazards. The company’s focus on infection control, clinical training, and integration with hospital workflows strengthens its position in both hospital and outpatient markets.
ICU Medical specializes in advanced IV therapy solutions with integrated safety features, including closed-system catheters and needle-free connectors. Its products are widely adopted in critical care and infusion therapy settings due to reliability, compliance with regulatory standards, and ease of use for healthcare professionals.
U.S. Peripheral Intravenous Catheters Industry News:
- In November 2023, BD (Becton, Dickinson and Company) introduced the PIVO Pro Needle-free Blood Collection Device, designed for use with integrated and long peripheral IV catheters, including the Nexiva Closed IV Catheter System. The device, which received FDA 510(k) clearance, supports BD’s One-Stick Hospital Stay initiative by enhancing patient safety, minimizing repeated venipunctures, and promoting wider adoption of integrated catheter systems, thereby reinforcing BD’s leadership in the U.S. vascular access market.
- In July 2022, B. Braun launched the Introcan Safety 2 IV Catheter featuring one-time blood control technology, designed to enhance clinician safety during IV access. The device minimizes the risk of needlestick injuries and blood exposure, supporting safer clinical workflows. This innovation reinforced B. Braun’s position in the U.S. market by driving the adoption of safety-engineered catheters and aligning with hospital and regulatory safety standards.
The U.S. peripheral intravenous catheters market research report includes an in-depth coverage of the industry with estimates and forecasts in terms of revenue in USD Million from 2021 – 2034 for the following segments:
Market, By Product
- Short PIVC
- Integrated/closed PIVC
Market, By Technology
- Safety PIVC
- Conventional PIVC
Market, By Lumen
- Single lumen
- Double lumen
- Multi-lumen
Market, By End Use
- Hospitals
- Ambulatory surgical centers
- Other end use
The above information is provided for the following zones and states:
- East North Central
- Illinois
- Indiana
- Michigan
- Ohio
- Wisconsin
- West South Central
- Arkansas
- Louisiana
- Oklahoma
- Texas
- South Atlantic
- Delaware
- Florida
- Georgia
- Maryland
- North Carolina
- South Carolina
- Virginia
- West Virginia
- Washington, D.C.
- Northeast
- Connecticut
- Maine
- Massachusetts
- New Hampshire
- Rhode Island
- Vermont
- New Jersey
- New York
- Pennsylvania
- East South Central
- Alabama
- Kentucky
- Mississippi
- Tennessee
- West North Central
- Iowa
- Kansas
- Minnesota
- Missouri
- Nebraska
- North Dakota
- South Dakota
- Pacific Central
- Alaska
- California
- Hawaii
- Oregon
- Washington
- Mountain States
- Arizona
- Colorado
- Utah
- Nevada
- New Mexico
- Idaho
- Montana
- Wyoming
Frequently Asked Question(FAQ) :
What are the upcoming trends in the U.S. peripheral intravenous catheters industry?
Key trends include the adoption of safety-engineered and antimicrobial-coated catheters, the integration of smart technology, and a focus on minimally invasive, patient-friendly designs.
Who are the key players in the U.S. peripheral intravenous catheters market?
Key players include Access Vascular, AngioDynamics, B. Braun, Becton, Dickinson and Company, DELTA MED, ICU Medical, Lineus Medical, MEDEREN, MedSource Labs, and Retractable Technologies.
What was the valuation of the safety PIVC segment in 2024?
The safety PIVC segment generated USD 1.4 billion in 2024 and is expected to grow at a CAGR of 6.8% during the forecast period.
Which region leads the U.S. peripheral intravenous catheters market?
The South Atlantic zone led the market with a 19.4% share in 2024, driven by a high concentration of healthcare facilities and increasing adoption of advanced medical devices.
What was the market size of the U.S. peripheral intravenous catheters market in 2024?
The market size was valued at USD 1.9 billion in 2024, with a CAGR of 6.7% projected through 2034, driven by the rising prevalence of chronic diseases, increasing hospitalizations, and advancements in catheter technology.
How much revenue did the short PIVC segment generate in 2024?
The short PIVC segment accounted for 63.9% of the market share in 2024, driven by its widespread use in hospitals and outpatient settings.
What is the projected size of the U.S. peripheral intravenous catheters market in 2025?
The market is anticipated to reach USD 2.1 billion in 2025.
What is the projected value of the U.S. peripheral intravenous catheters market by 2034?
The market is expected to reach USD 3.8 billion by 2034, fueled by the expansion of outpatient and home infusion therapies and the adoption of safety-engineered devices.
U.S. Peripheral Intravenous Catheters Market Scope
Related Reports


