Summary
Table of Content

U.S. Pancreatic Cancer Diagnostic Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
U.S. Pancreatic Cancer Diagnostic Market Size
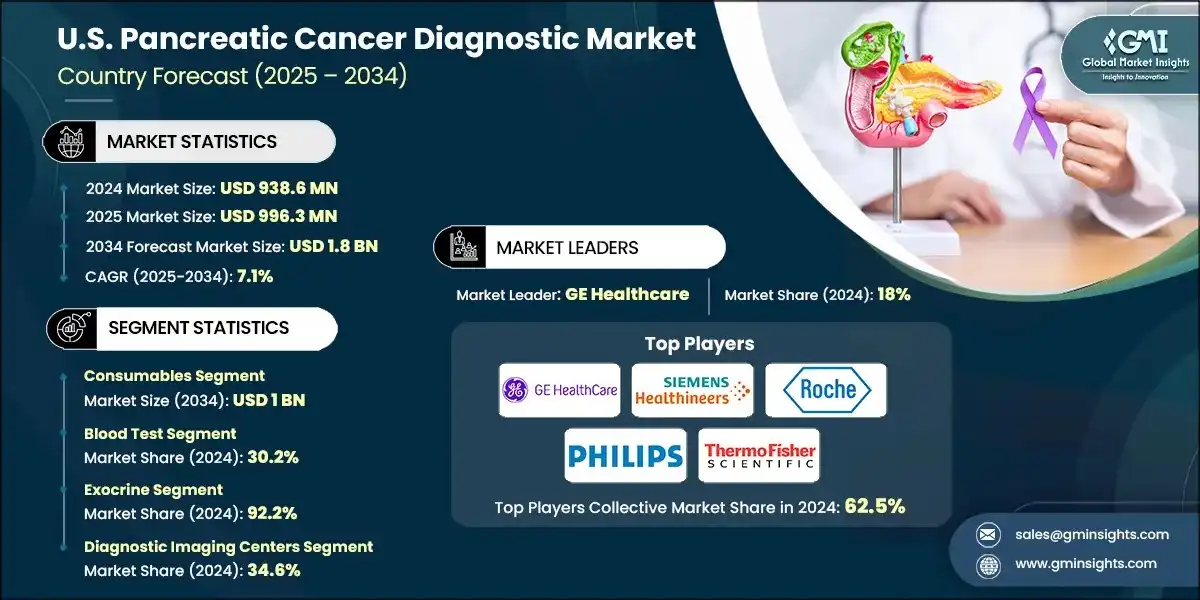
The U.S. pancreatic cancer diagnostic market was estimated at USD 938.6 million in 2024. The market is expected to grow from USD 996.3 million in 2025 to USD 1.8 billion in 2034, growing at a CAGR of 7.1%, according to the latest report published by Global Market Insights Inc. The market is experiencing steady growth, fueled by the rising prevalence of pancreatic cancer in the U.S., advancements in diagnostic technologies, increasing demand for early detection, and a growing elderly population.
To get key market trends
In the U.S., advanced tools such as imaging, biopsies, blood-based biomarkers, and molecular techniques including liquid biopsies and next-generation sequencing are integral to pancreatic cancer diagnostics. Companies such as F. Hoffmann-La Roche, Thermo Fisher Scientific, GE Healthcare, Siemens Healthineers, and Philips are investing in technologies to improve early detection and diagnostic accuracy. These efforts aim to enhance survival rates for this highly lethal disease.
The market grew from USD 779.9 million in 2021 to USD 882.4 million in 2023. The U.S. pancreatic cancer diagnostic market is expanding rapidly due to increasing awareness of early detection, national screening initiatives, and a shift toward preventive healthcare. Since the disease is often diagnosed at a late stage, there is growing demand for tools that enable earlier and more accurate identification. Efforts to streamline clinical workflows and improve patient outcomes are accelerating adoption. Policy support and integration into proactive cancer care are further driving market growth.
The rising number of pancreatic cancer cases in the U.S. is a major factor driving the growth of the market. For instance, according to data from the World Cancer Research Fund, approximately 60,127 new cases of pancreatic cancer were reported in the U.S. in 2022, with 31,598 cases among men and 28,529 among women. These alarming figures highlight the urgent need for robust diagnostic solutions that can detect pancreatic cancer at earlier stages, when treatment is more effective.
Furthermore, the U.S. pancreatic cancer diagnostic market is experiencing notable growth, primarily driven by the country’s growing geriatric population. For example, the U.S. Population Reference Bureau (PRB) projects that the number of Americans aged 65 and older will increase from 58 million in 2022 to 82 million by 2050. This age group’s share of the total population is expected to grow from 17% to 23%. This demographic shift is anticipated to significantly boost the demand for routine diagnostic procedures, including those aimed at the early detection of pancreatic cancer. As older adults require more frequent medical monitoring, healthcare providers are increasingly incorporating pancreatic cancer screening into preventive care strategies. This trend is further driving the growth of diagnostic services and devices across the country.
Pancreatic cancer diagnostics include a variety of medical tests, imaging technologies, and molecular tools designed to detect, confirm, and monitor the disease. These diagnostics are vital for identifying cancerous cells in the pancreas, determining the stage of the disease, and guiding treatment decisions effectively.
U.S. Pancreatic Cancer Diagnostic Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 938.6 Million |
| Market Size in 2025 | USD 996.3 Million |
| Forecast Period 2025 - 2034 CAGR | 7.1% |
| Market Size in 2034 | USD 1.8 billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of pancreatic cancer in the U.S. | Drives demand for early and accurate diagnostic tools to improve survival outcomes. |
| Growing demand for early detection | Accelerates innovation in non-invasive and biomarker-based diagnostic tools. |
| Rising geriatric population | Fuels demand for age-specific diagnostic procedures due to higher susceptibility to pancreatic cancer. |
| Advancements in diagnostic technologies | Encourages integration of screening protocols in routine care, especially for high-risk populations. |
| Pitfalls & Challenges | Impact |
| Stringent regulatory scenarios | Slows the approval and adoption of innovative diagnostic tools, especially in decentralized care settings. |
| High cost of diagnostic tests | Limits access for uninsured or underinsured populations, affecting early detection efforts. |
| Opportunities: | Impact |
| Integration of precision medicine and genomic testing | Enables molecular profiling and personalized diagnostic strategies for early-stage detection. |
| Market Leaders (2024) | |
| Market Leaders |
18% |
| Top Players |
Collective market share in 2024 is 62.5% |
| Competitive Edge |
|
| Regional Insights | |
| Future outlook |
|
What are the growth opportunities in this market?
U.S. Pancreatic Cancer Diagnostic Market Trends
- Technological advancements are reshaping the U.S. pancreatic cancer diagnostic landscape, addressing long-standing challenges in early detection caused by asymptomatic progression and the pancreas's deep anatomical location.
- AI-powered imaging systems are revolutionizing diagnostic precision. Researchers at the Mayo Clinic Comprehensive Cancer Center have developed an artificial intelligence (AI) model that shows potential for autonomously detecting pancreatic cancer on standard CT scans when surgery can still offer a curative option, highlighting its transformative role in early screening.
- Additionally, liquid biopsy technologies are gaining traction across U.S. clinical settings due to their non-invasive nature and high sensitivity. These tests analyze ctDNA, CTCs, and exosomes, providing real-time insights into tumor biology and enabling earlier intervention.
- Innovative solutions such as Thermo Fisher Scientific’s TaqMan Liquid Biopsy dPCR Assays and QIAGEN’s QIAamp ccfDNA/RNA Kit are facilitating sensitive mutation detection and comprehensive genetic profiling.
- Furthermore, point-of-care diagnostic tools are emerging as critical solutions in low-resource settings, offering rapid testing and immediate results to reduce diagnostic delays and improve access to care in the U.S.
U.S. Pancreatic Cancer Diagnostic Market Analysis
Learn more about the key segments shaping this market
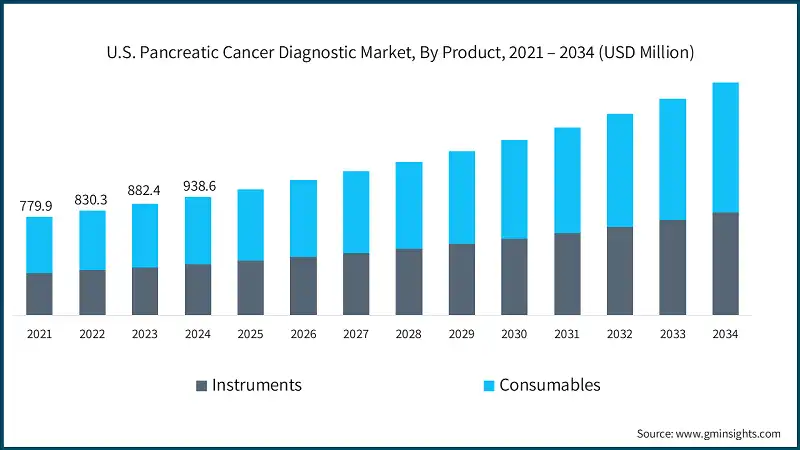
Based on the product, the U.S. pancreatic cancer diagnostic market is segmented into instruments and consumables. The consumables segment led this market in 2024 and was valued at USD 534.1 million in 2024 and is projected to reach USD 1 billion by 2034, growing at a CAGR of 6.9%. This growth is due to the continuous need for repeat testing, rising adoption of personalized medicine, and increasing awareness and screening initiatives taken by the government. In comparison, the instruments segment, valued at USD 404.5 million in 2024, is expected to grow to USD 811.9 million by 2034, with a slightly higher CAGR of 7.3%, supported by continuous technological innovations, increasing adoption of AI-powered imaging systems, and rising demand for advanced diagnostic equipment in hospitals and diagnostic imaging centers.
- The instruments segment forms the backbone of the U.S. pancreatic cancer diagnostics market, encompassing cutting-edge imaging systems, endoscopic tools, and molecular diagnostic, which is estimated to reach USD 40.4 billion by 2034. These technologies are vital for accurate detection, staging, and monitoring of pancreatic cancer, a disease often diagnosed late due to its asymptomatic nature.
- In the U.S., demand for advanced diagnostic instruments is accelerating, driven by innovations in imaging and molecular profiling. For example, GE Healthcare’s SIGNA Premier 3T MRI system, which is FDA-cleared and widely adopted across U.S. institutions, delivers ultra-high-resolution imaging with AIR Recon DL technology. This system enables detailed visualization of pancreatic structures while enhancing patient comfort through its silent scanning and wide-bore design.
- Similarly, Siemens Healthineers’ SOMATOM Force CT scanner leverages dual-source imaging and AI-powered automation to reduce scan time and radiation exposure. Its Turbo Flash mode and Vectron X-ray tube allow for ultra-low-dose, high-resolution imaging, even in challenging cases such as pediatric or obese patients.
- In addition, due to continuous progress in AI, imaging precision, and molecular diagnostics, the instruments segment is poised to remain a key growth driver in the U.S. pancreatic cancer diagnostics market.
Based on the test type, the U.S. pancreatic cancer diagnostic market is segmented into imaging tests, biopsy, blood tests, and other test types. Further, the blood test segment is bifurcated into liver function tests, tumor markers, and other blood tests. The blood test segment accounted for the highest market share of 30.2% in 2024.
- The blood test segment of the U.S. pancreatic cancer diagnostic market is expanding rapidly, driven by its non-invasive nature, ease of administration, and growing potential for early detection.
- Traditional biomarkers such as CA 19-9 and CEA remain widely used for monitoring disease progression. However, their limited sensitivity in early-stage detection has led to the development of more advanced molecular diagnostics.
- Innovations include the IMMray PanCan-d test, which utilizes a multiplex biomarker signature to detect pancreatic cancer at an early stage. Another promising development is a lab-on-a-chip plasma test that identifies electrical biomarkers in blood and is currently undergoing human trials.
- Additionally, liquid biopsy platforms analyzing circulating tumor DNA (ctDNA), exosomes, and microRNA are gaining traction. These technologies provide real-time insights into tumor genetics and heterogeneity, enabling personalized treatment strategies and driving market growth.
Based on the cancer type, the U.S. pancreatic cancer diagnostic market is segmented into exocrine and endocrine. Further, the exocrine segment is bifurcated into adenocarcinoma, colloid carcinoma, adenosquamous carcinoma, and squamous cell carcinoma. The exocrine segment accounted for the highest market share of 92.2% in 2024.
- The U.S. pancreatic cancer diagnostic market is witnessing steady growth, primarily due to the rising incidence of exocrine pancreatic cancers, which account for over 85% of all pancreatic malignancies. These include aggressive subtypes such as adenocarcinoma, colloid carcinoma, adenosquamous carcinoma, and squamous cell carcinoma, all originating in the exocrine glands responsible for producing digestive enzymes.
- Among these, colloid carcinoma, also known as mucinous non-cystic carcinoma, is a rare but distinct subtype that typically arises from intraductal papillary mucinous neoplasms (IPMNs). It is characterized by abundant extracellular mucin and a relatively favorable prognosis compared to conventional pancreatic ductal adenocarcinoma (PDAC).
- Markers such as MUC2, MUC5AC, and CDX2 are commonly expressed and help distinguish colloid carcinoma from PDAC and other exocrine subtypes. These markers are increasingly integrated into diagnostic workflows at major academic and cancer research centers, enhancing diagnostic specificity and guiding personalized treatment strategies.
Learn more about the key segments shaping this market
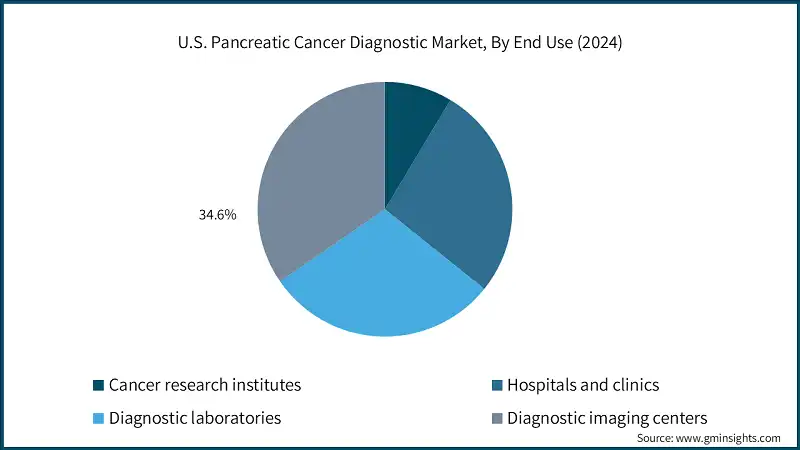
Based on end use, the U.S. pancreatic cancer diagnostic market is segmented into cancer research institutes, hospitals and clinics, diagnostic laboratories, and diagnostic imaging centers. In 2024, diagnostic imaging centers held the largest share of about 34.6%.
- Diagnostic imaging centers have the presence of advanced imaging systems, which offer high-resolution visualizations, which is beneficial in diagnosing cancer in pancreatic tissues. As a result, they are often the first point of contact for diagnosing malignant disorders.
- Similarly, in 2024, diagnostic laboratories held a share of 29.7%. Diagnostic laboratories are a crucial end-user segment in the U.S. pancreatic cancer diagnostics market, serving as specialized hubs for biomarker analysis, molecular profiling, and advanced imaging interpretation.
- The growing prevalence of pancreatic cancer, often diagnosed at a late stage, has increased the demand for specialized diagnostic capabilities. These laboratories are equipped with sophisticated technologies such as next-generation sequencing (NGS), PCR-based platforms, and liquid biopsy analyzers, enabling the identification of genetic mutations, circulating tumor DNA (ctDNA), and protein biomarkers such as CA 19-9.
- In addition, diagnostic laboratories play a vital role in clinical trials and translational research. Strategic collaborations between diagnostic labs and pharmaceutical companies are fostering innovation in biomarker discovery and validation, thereby driving market growth.
U.S. Pancreatic Cancer Diagnostic Market Share
- The top five players F. Hoffmann-La Roche, Thermo Fisher Scientific, GE Healthcare, Siemens Healthineers, and Koninklijke Philips collectively hold 62.5% of the U.S. pancreatic cancer diagnostics market. These companies continue to strengthen their market positions through innovation, regulatory compliance, and strategic collaborations. Significant investments are being directed toward next-generation biopsy tools that enhance precision, enable real-time imaging, and support minimally invasive procedures.
- Koninklijke Philips significantly contributes to the U.S. market through its IntelliSpace Genomics platform, developed in collaboration with Memorial Sloan Kettering Cancer Center. This platform supports precision diagnostics by enabling single-cell-level genomic analysis, helping uncover the molecular drivers of pancreatic cancer. The partnership aims to improve diagnostic accuracy and inform personalized treatment strategies by integrating advanced analytics and next-generation sequencing technologies.
- Manufacturers across the region are also adopting value-based pricing strategies to enhance accessibility in cost-sensitive markets. They are launching AI-assisted platforms for pancreatic cancer screening, featuring real-time molecular profiling, automated lesion detection, and integrated reporting tools, thereby expanding access to precision diagnostics in outpatient and decentralized care settings.
- Emerging trends in the U.S. pancreatic cancer diagnostics market include the development of minimally invasive liquid biopsy tools, AI-powered imaging systems, and workflow-integrated platforms designed for early detection and personalized oncology. Technologies such as circulating tumor DNA (ctDNA) assays, contrast-enhanced endoscopic ultrasound, and radiomics-based AI models are significantly enhancing diagnostic precision, accessibility, and patient outcomes.
U.S. Pancreatic Cancer Diagnostic Market Companies
Few of the prominent players operating in the U.S. pancreatic cancer diagnostic industry include:
- Abbott Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Boston Scientific
- Canon
- Danaher
- F Hoffmann-La Roche
- GE Healthcare
- Illumina
- Koninklijke Philips
- Myriad Genetics
- Olympus
- QIAGEN
- Siemens Healthineers
- Sysmex
- Thermo Fisher Scientific
GE Healthcare holds a significant share in the U.S. pancreatic cancer diagnostic market through its comprehensive product portfolio. GE Healthcare emphasizes R&D and product development offerings with advanced imaging capabilities, ergonomic designs, and AI-powered diagnostic tools.
Olympus Corporation is recognized for pioneering innovative, patient-centric diagnostic technologies that enable early and accurate detection of pancreatic cancer, enhance clinical confidence, and improve patient outcomes.
Siemens Healthineers holds a significant share in the market through its comprehensive product portfolio. It includes the FDA-cleared NAEOTOM Alpha Photon-Counting CT Scanner and Atellica CI Analyzer, among others.
U.S. Pancreatic Cancer Diagnostic Industry News:
- In July 2025, Olympus Corporation launched the EU-ME3 Ultrasound Processor in the U.S., a compact, integrated system combining EUS and EBUS capabilities with high-resolution imaging and advanced functionality for hepatobiliary-pancreatic and pulmonary procedures. This innovation enhances Olympus’s market reach, boosts clinical efficiency, and strengthens its position as a leader in medical imaging technology.
The U.S. pancreatic cancer diagnostic market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Product
- Instruments
- Consumables
Market, By Test Type
- Imaging test
- CT scan
- MRI
- Ultrasound
- PET
- Other imaging tests
- Biopsy
- Blood test
- Liver function tests
- Tumor markers
- Other blood tests
- Other test types
Market, By Cancer Type
- Exocrine
- Adenocarcinoma
- Colloid carcinoma
- Adenosquamous carcinoma
- Squamous cell carcinoma
- Endocrine
Market, By End Use
- Cancer research institutes
- Hospitals and clinics
- Diagnostic laboratories
- Diagnostic imaging centers
Frequently Asked Question(FAQ) :
Who are the key players in the U.S. pancreatic cancer diagnostic market?
Key players include GE Healthcare, Siemens Healthineers, F. Hoffmann-La Roche, Thermo Fisher Scientific, Koninklijke Philips, QIAGEN, Illumina, Abbott, Olympus, Agilent Technologies, Boston Scientific, Myriad Genetics, Sysmex, Canon, Becton Dickinson, and Danaher.
What are the upcoming trends in the pancreatic cancer diagnostic industry?
Key trends include adoption of AI-powered imaging, liquid biopsy tools, next-generation sequencing, and workflow-integrated diagnostic platforms for early detection.
Which end-use segment dominates the market?
Diagnostic imaging centers led with a 34.6% share in 2024, owing to advanced imaging systems and their role as first-line diagnostic facilities.
Which test type leads the pancreatic cancer diagnostic market?
Blood tests accounted for the highest share at 30.2% in 2024, driven by ease of use, non-invasive nature, and increasing adoption of liquid biopsy platforms.
What was the valuation of the instruments segment?
The instruments segment was valued at USD 404.5 million in 2024 and is expected to reach USD 811.9 million by 2034.
What is the projected size of the pancreatic cancer diagnostic industry in 2025?
The U.S. pancreatic cancer diagnostic market is expected to reach USD 996.3 million in 2025.
How much revenue did the consumables segment generate?
The consumables segment generated USD 534.1 million in 2024, projected to reach USD 1 billion by 2034.
What is the market size of the U.S. pancreatic cancer diagnostic in 2024?
The market size was USD 938.6 million in 2024.
What is the projected value of the U.S. pancreatic cancer diagnostic market by 2034?
The market is expected to reach USD 1.8 billion by 2034, growing at a CAGR of 7.1%.
U.S. Pancreatic Cancer Diagnostic Market Scope
Related Reports


