Summary
Table of Content

U.S. Epilepsy Treatment Drugs Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
U.S. Epilepsy Treatment Drugs Market Size
The U.S. epilepsy treatment drugs market was valued at USD 3.1 billion in 2024 and is projected to grow from USD 3.2 billion in 2025 to USD 4.7 billion by 2034, expanding at a CAGR of 4.4% according to the latest report published by Global Market Insights Inc.
To get key market trends
The growth of the U.S. epilepsy treatment drugs market is driven by several factors, including the rising prevalence of epilepsy in the U.S., increasing investments in research and development activities, growing demand for novel treatments, and rising awareness and early diagnosis. Key players in this market include Pfizer, Novartis AG, GSK (GlaxoSmithKline), and Jazz Pharmaceuticals.
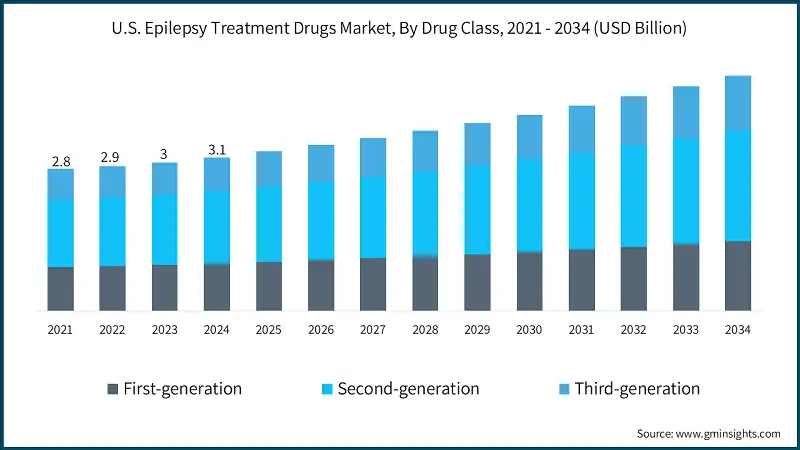
The market value increased from USD 2.8 billion in 2021 to USD 3 billion in 2023. The rising prevalence of epilepsy in the U.S. is a major driver of growth in the epilepsy treatment drugs market. As more individuals are diagnosed with this neurological disorder, the demand for effective and accessible medications continues to increase. For instance, according to the Centers for Disease Control and Prevention (CDC), approximately 2.9 million U.S. adults aged 18 and older reported having active epilepsy during 2021–2022. This statistic underscores the widespread nature of the condition and the critical need for advanced treatment options. Thus, the growing incidence of epilepsy among individuals is accelerating market expansion for epilepsy treatment drugs.
Increasing investment in research and development is a significant factor driving the growth of the epilepsy treatment drugs market. As pharmaceutical companies and research institutions allocate more resources to understanding neurological disorders, the development of advanced and more effective anti-epileptic drugs becomes possible. These investments foster innovation in drug formulations, improve safety profiles, minimize side effects, and enhance patient outcomes. The steady flow of funding into research and development reflects a strong commitment to addressing complex conditions such as epilepsy through scientific advancements.
For example, according to the National Center for Science and Engineering Statistics, research and development expenditure in the U.S. reached USD 892 billion in 2022 and is estimated to rise to USD 940 billion in 2023. This surge in research and development spending plays a pivotal role in shaping the future of epilepsy care and driving innovation in treatment options.
Epilepsy treatment drugs, also known as anti-seizure or anticonvulsant medications, are pharmaceutical agents used to control and prevent seizures in individuals diagnosed with epilepsy. These drugs work by stabilizing electrical activity in the brain, limiting the spread of abnormal signals that cause seizures. They are considered the first-line and most common form of treatment for epilepsy.
U.S. Epilepsy Treatment Drugs Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 3.1 Billion |
| Market Size in 2025 | USD 3.2 Billion |
| Forecast Period 2025 - 2034 CAGR | 4.4% |
| Market Size in 2034 | USD 4.7 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising prevalence of epilepsy in U.S. | Drives increased demand for effective and accessible treatment options. |
| Increasing investments in research and development activities | Accelerates innovation and introduction of advanced therapies. |
| Rising demand for novel treatment for epilepsy | Fuels market growth through adoption of cutting-edge and personalized solutions. |
| Growing awareness and early diagnosis | Expanding the patient pool and boosting early intervention, enhancing market penetration. |
| Pitfalls & Challenges | Impact |
| High treatment costs | Limits patient access and slows market growth due to affordability concerns. |
| Patent expiration | Intensifies generic competition, reducing revenue for branded drug manufacturers. |
| Opportunities: | Impact |
| Advancements in drug development | Enable the introduction of more effective and safer epilepsy treatments, driving innovation and market expansion. |
| Market Leaders (2024) | |
| Market Leaders |
10% market share |
| Top Players |
Collective market share in 2024 is 42% |
| Competitive Edge |
|
| Future outlook |
|
What are the growth opportunities in this market?
U.S. Epilepsy Treatment Drugs Market Trends
- Growing awareness and early diagnosis of epilepsy are important factors driving the expansion of the epilepsy treatment drugs market. Public education campaigns and advocacy initiatives have significantly improved understanding of the condition, reducing stigma and encouraging timely medical intervention.
- For example, Epilepsy Alliance America’s theme for Epilepsy Awareness Month in November 2024, Empowering Every Journey, emphasizes programs, resources, and advocacy efforts that ensure individuals with epilepsy and their caregivers receive support and guidance. These initiatives foster community engagement and promote seizure first-aid training, ultimately leading to earlier diagnosis and better treatment outcomes.
- Additionally, increased awareness not only accelerates patient access to care but also boosts demand for effective and safe anti-epileptic medications, strengthening the importance of innovation in drug development.
- Awareness programs provide training on seizure recognition and first aid, encouraging timely medical intervention. This proactive approach leads to earlier diagnosis and treatment, improving patient outcomes and increasing demand for effective anti-epileptic drugs.
- Moreover, growing awareness translates into higher diagnosis rates, which directly drives the need for advanced and safer treatment options. As more people recognize symptoms and seek medical help promptly, pharmaceutical companies benefit from increased demand, fueling innovation and market expansion.
U.S. Epilepsy Treatment Drugs Market Analysis
Learn more about the key segments shaping this market
Based on drug class, the market is segmented into first-generation, second-generation, and third generation. The second-generation segment dominated the market with the largest revenue share of 47.3% in 2024 and is expected to grow at a CAGR of 4.4% over the forecast period.
- Second-generation anti-epileptic drugs (AEDs) are increasingly shaping the management of epilepsy due to their superior safety, tolerability, and patient-friendly profiles. These medications have fewer side effects, reduced drug-drug interactions, and improved compliance compared to first-generation agents, making them ideal for long-term treatment plans and combination therapies.
- Regulatory bodies such as the U.S. FDA continue to approve new-generation therapeutic agents for multiple indications, including epilepsy-related complications and neuropathic pain, reinforcing their clinical value and global market potential.
- Additionally, advanced formulations such as extended-release versions and simplified administration methods enhance patient experience and adherence. Their versatility in treating various neurological conditions positions second-generation AEDs as a critical driver of innovation and growth in the epilepsy treatment drugs market.
Based on type, the U.S. epilepsy treatment drugs market is bifurcated into branded and generics. The branded segment held a revenue of USD 946.7 million in 2024 and is expected to reach USD 1.5 billion by 2034.
- Leading pharmaceutical companies are increasingly prioritizing branded epilepsy medications that offer improved safety, reduced side effects, and enhanced effectiveness. This shift addresses the needs of patients unresponsive to standard therapies, particularly those with drug-resistant epilepsy, making branded drugs a preferred choice in clinical practice.
- Examples include Pfizer’s Lyrica (pregabalin), UCB’s Keppra (levetiracetam) and Briviact (brivaracetam), and SK Biopharmaceuticals’ Xcopri (cenobamate).
- Regulatory approvals and ongoing innovation are playing important role in strengthening the branded epilepsy drug segment. Frequent approvals by major bodies such as the U.S. FDA validate the safety, efficacy, and clinical relevance of these therapies, which builds trust among healthcare providers and patients.
- Further, with rising demand for effective therapies and continued investment in advanced formulations, the branded drug segment is poised for significant growth in the epilepsy treatment market.
Based on route of administration, the U.S. epilepsy treatment drugs market is segmented into oral, nasal, injectable, and rectal. The oral segment dominated the market with the largest revenue of USD 1.9 billion in 2024 and is expected to grow at a CAGR of 4.6% over the forecast period.
- Oral extended-release formulations are gaining traction in epilepsy treatment due to their ability to maintain steady drug concentrations over time, reducing dosing frequency and improving patient adherence.
- The growing variety of oral anti-epileptic drug (AED) formulations caters to different age groups and seizure types, offering a convenient alternative for patients who struggle with injections or complex dosing schedules.
- For instance, Supernus Pharmaceuticals’ Trokendi XR is approved for managing partial seizures, generalized tonic-clonic seizures, and Lennox-Gastaut syndrome in children over 10 and adults.
- Further, extended-release oral drugs are generally well tolerated and associated with fewer drug-drug interactions compared to standard formulations. These clinical advantages, combined with strong patient preference, are expected to drive continued growth in this segment.
Based on age group, the U.S. epilepsy treatment drugs market is bifurcated into pediatric and adult. The pediatric segment is fastest growing in the market with the revenue of USD 844.4 million in 2024 and is expected to grow at a CAGR of 4.6% over the forecast period.
- The pediatric segment of the epilepsy treatment market is growing rapidly, driven by early diagnosis and the development of child-friendly drug formulations. More children are being diagnosed at younger ages, prompting pharmaceutical companies to create safe and easy-to-administer options such as liquid formulations and sprinkle capsules, which improve treatment adherence and outcomes.
- For example, according to the Epilepsy Foundation, approximately 470,000 children under the age of 14 live with epilepsy, highlighting the significant need for specialized therapies.
- Additionally, the rise in epilepsy cases linked to genetic and developmental disorders in younger age groups is fueling demand for targeted treatments. With growing attention to early intervention, child-specific drug formats, and innovative therapies, the pediatric segment is expected to experience sustained growth in the coming years.
Based on seizure type, the U.S. epilepsy treatment drugs market is segmented into focal seizure, generalized seizure, and combined seizure. The generalized seizure segment is fastest growing in the market with the revenue share of 29.7% in 2024 and is expected to grow at a CAGR of 4.8% over the forecast period.
- Generalized seizures are a major category of epileptic seizures that affect both hemispheres of the brain simultaneously, leading to widespread electrical disturbances. These seizures include subtypes such as tonic-clonic, absence, and myoclonic seizures, each requiring tailored therapeutic approaches.
- Treatment typically involves broad-spectrum anti-epileptic drugs (AEDs) that can control multiple seizure types, such as valproate, lamotrigine, and levetiracetam. Second-generation AEDs such as Briviact (brivaracetam) and extended-release formulations have gained prominence for their improved safety profiles, reduced side effects, and better patient adherence.
- Furthermore, the management of generalized seizures focuses on achieving optimal seizure control while minimizing cognitive and behavioral impacts, which is critical for long-term quality of life. With ongoing innovation and regulatory approvals, newer therapies are being introduced to address drug-resistant cases and improve tolerability, making this segment a key driver in the epilepsy treatment drugs market.
Learn more about the key segments shaping this market
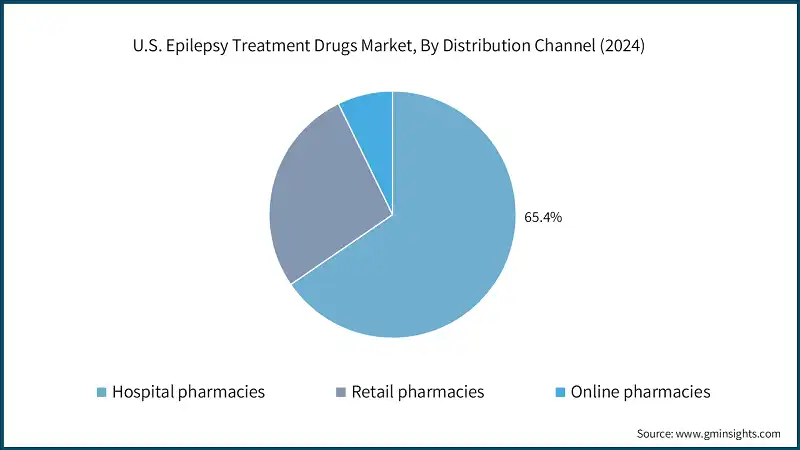
Based on distribution channel, the U.S. epilepsy treatment drugs market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The retail pharmacies segment held market revenue of USD 840.7 million in 2024 and is expected to grow at a CAGR of 4.7% over the forecast period.
- Retail pharmacies play an important role in ensuring accessibility and convenience for epilepsy treatment drugs, serving as a primary channel for patients managing chronic conditions. They offer a wide range of options, including branded and generic anti-epileptic drugs (AEDs), with generics providing comparable effectiveness at significantly lower costs, making treatment more affordable for many patients.
- Modern retail pharmacies also provide added services such as medication consultations, refill reminders, and adherence programs, which improve treatment compliance and outcomes.
- Additionally, large pharmacy chains are expanding their reach through digital platforms, offering home delivery and online consultations to enhance patient convenience. As demand for reliable and easily accessible medication sources grows, retail pharmacies are expected to maintain a dominant position in the epilepsy treatment drug distribution landscape.
U.S. Epilepsy Treatment Drugs Market Share
- In the U.S. epilepsy treatment drugs industry, leading pharmaceutical companies such as UCB SA, Pfizer, Novartis AG, GSK (GlaxoSmithKline), and Jazz Pharmaceuticals are strengthening their presence through diverse product portfolios, robust regulatory compliance, and continuous innovation. Collectively, these top five players account for about 42% of the market share. Their growth is further fueled by strategic collaborations with research organizations, healthcare providers, and distributors, enabling broader access to advanced treatment options.
- These companies are actively investing in the development of both branded and generic anti-epileptic drugs, catering to diverse patient needs across different age groups and seizure types. By focusing on improved drug formulations, faster approvals, and patient-friendly delivery methods, they continue to shape the competitive landscape of the epilepsy treatment market.
- Emerging players in the U.S. epilepsy treatment drug market are adopting diverse strategies to strengthen their position. These include utilizing advanced drug delivery technologies, developing next-generation formulations with improved efficacy and safety, and integrating AI-driven platforms for personalized treatment planning. Many companies are focusing on precision medicine approaches for seizure control and forming strategic partnerships with hospitals, research institutions, and established pharmaceutical firms to address high development costs and regulatory challenges.
U.S. Epilepsy Treatment Drugs Market Companies
Few of the prominent players operating in the U.S. epilepsy treatment drugs industry include:
- AbbVie
- Bausch Health Companies
- Dr. Reddy’s Laboratories
- Eisai
- GSK
- Jazz Pharmaceuticals
- Lupin Pharmaceuticals
- Neurelis
- Novartis
- Pfizer
- Sanofi
- SK Biopharmaceuticals
- Sumitomo Pharma
- Sun Pharmaceutical Industries
- UCB
- Eisai
Eisai is a prominent player in epilepsy care, recognized for its focus on neurological research and patient-centered therapies. Its flagship drug, Fycompa (perampanel), treats partial-onset and generalized tonic-clonic seizures in both adults and pediatric patients.
Dr. Reddy’s Laboratories, through its subsidiary Betapharm Arzneimittel GmbH, plays a vital role in providing high-quality generic medicines for epilepsy treatment.
Pfizer is driving advancements in epilepsy care through the development of second- and third-generation anti-epileptic drugs, strategic product launches, and sustained investment in neuroscience. Within its product portfolio, Zarontin (Ethosuximide Capsules) plays a key role in managing absence (petit mal) epilepsy, strengthening Pfizer’s commitment to addressing diverse patient needs.
North America Epilepsy Treatment Drugs Industry News:
- In April 2025, Neurelis received FDA approval for VALTOCO (diazepam nasal spray) for short-term treatment of seizure clusters in patients aged 2 years and older. Recognized as clinically superior to rectal diazepam gel and granted orphan drug exclusivity, this approval strengthens Neurelis’ position in emergency epilepsy care and enhances its credibility with healthcare providers.
The U.S. epilepsy treatment drugs market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Drug Class
- First-generation
- Second-generation
- Third-generation
Market, By Type
- Branded
- Generics
Market, By Route of Administration
- Oral
- Nasal
- Injectable
- Rectal
Market, By Age Group
- Pediatric
- Adult
Market, By Seizure Type
- Focal seizure
- Generalized seizure
- Combined seizure
Market, By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
Frequently Asked Question(FAQ) :
What was the revenue share of the second-generation drug class segment in 2024?
The second-generation drug class segment held the largest revenue share of 47.3% in 2024 and is projected to grow at a CAGR of 4.4% over the forecast period.
What was the valuation of the branded segment in 2024?
The branded segment generated USD 946.7 million in 2024 and is expected to reach USD 1.5 billion by 2034.
Who are the key players in the U.S. epilepsy treatment drugs market?
Key players include AbbVie, Bausch Health Companies, Dr. Reddy’s Laboratories, Eisai, GSK, Jazz Pharmaceuticals, Lupin Pharmaceuticals, Neurelis, Novartis, Pfizer, Sanofi, and SK Biopharmaceuticals.
Which age group is the fastest-growing segment in the U.S. epilepsy treatment drugs market?
The pediatric segment is the fastest-growing, with a revenue of USD 844.4 million in 2024 and an anticipated CAGR of 4.6% through the forecast period.
Which route of administration dominated the U.S. epilepsy treatment drugs market in 2024?
The oral route of administration dominated the market, generating USD 1.9 billion in 2024, with a projected CAGR of 4.6% over the forecast period.
What was the market size of the U.S. epilepsy treatment drugs market in 2024?
The market size was USD 3.1 billion in 2024, with a CAGR of 4.4% expected through 2034, driven by increased awareness, early diagnosis, and innovation in drug development.
What is the projected size of the U.S. epilepsy treatment drugs market in 2025?
The market is expected to reach USD 3.2 billion in 2025.
What is the projected value of the U.S. epilepsy treatment drugs market by 2034?
The market is expected to reach USD 4.7 billion by 2034, supported by growing demand for effective anti-epileptic medications and advancements in treatment options.
U.S. Epilepsy Treatment Drugs Market Scope
Related Reports


