Summary
Table of Content

Real World Evidence Solutions Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Real World Evidence Solutions Market Size
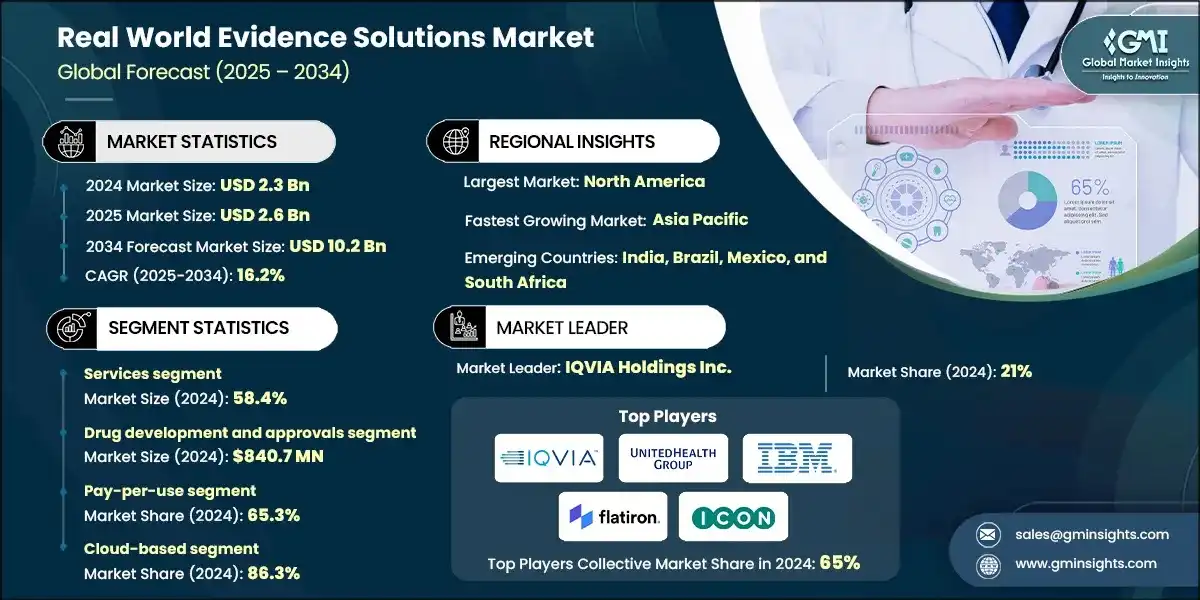
The global real world evidence solutions market was estimated at USD 2.3 billion in 2024. The market is expected to grow from USD 2.6 billion in 2025 to USD 10.2 billion in 2034, growing at a CAGR of 16.2%, according to the latest report published by Global Market Insights Inc. The growth of the market is largely influenced by the growing focus towards accelerating drug development and cost reduction, growing demand for real-time safety and efficacy monitoring of drugs and medical devices, increasing adoption of RWE Solutions for informed reimbursement decision-making.
To get key market trends
The market increased from USD 1.5 billion in 2021 to USD 2 billion in 2023. This growth was driven by the growing focus towards accelerating drug development and cost reduction, traditional drug development is slow and expensive, relying heavily on multi-phase clinical trials and regulatory reviews. RWE enables faster, data-driven decisions by integrating real-world data (RWD) from sources like EHRs, claims, and patient registries. This helps pharmaceutical companies identify viable candidates, validate efficacy across diverse populations, and monitor safety in real-world settings ultimately reducing trial size and cost.
Additionally, RWE is increasingly used in preclinical research to supplement animal studies with historical clinical data. This helps detect early safety signals and optimize dosing strategies. Regulatory bodies like the FDA and EMA now recognize RWE’s value, encouraging biopharma firms to invest in RWE platforms for faster approvals and reduced trial expenditures.
The increasing adoption of RWE solutions for informed reimbursement decision-making, as healthcare systems increasingly shift toward value-based care, the adoption of Real World Evidence (RWE) solutions is becoming essential for informed reimbursement decisions. Health Technology Assessment (HTA) bodies and payers are leveraging RWE to evaluate how therapies perform in real-world clinical settings, beyond the controlled conditions of randomized clinical trials. This enables more transparent, cost-effective, and patient-centered decision-making.
To fully integrate RWE into reimbursement frameworks, HTA bodies are building strong infrastructures, including analytical capabilities and standardized methodologies. The European Medicines Agency (EMA), for instance, has developed the DARWIN EU network to generate timely, reliable RWE for regulatory and payer decisions. Such initiatives enhance consistency and transparency, fostering trust among stakeholders and accelerating the adoption of RWE platforms across global healthcare systems.
Real world evidence solutions represent a diverse array of methodologies, technologies, and services that are designed to generate valuable insights into various aspects of healthcare. These solutions leverage real-world data, which is collected from sources outside the controlled environment of traditional clinical trials, to provide a more comprehensive understanding of healthcare outcomes, treatment effectiveness, and patient experiences.
Real World Evidence Solutions Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 2.3 Billion |
| Market Size in 2025 | USD 2.6 Billion |
| Forecast Period 2025 – 2034 CAGR | 16.2% |
| Market Size in 2034 | USD 10.2 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Growing focus towards accelerating drug development and cost reduction | Drives demand for efficient RWE platforms to streamline trials and reduce research and development expenses. |
| Growing demand for real-time safety and efficacy monitoring of drugs and medical devices | Boosts adoption of RWE tools for post-market surveillance and regulatory compliance. |
| Increasing adoption of RWE Solutions for informed reimbursement decision-making | Encourages payers and providers to rely on evidence-based value assessments, expanding market scope. |
| Increasing adoption of data analytics services in clinical decision making | Enhances the utility of RWE in personalized medicine and treatment optimization. |
| Pitfalls & Challenges | Impact |
| Lack of standardization in integration and interoperability of real-world data | Limits scalability and consistency of RWE insights across platforms and stakeholders. |
| Shortage of skilled professionals | Slows adoption and innovation due to gaps in data science, regulatory, and clinical expertise. |
| Opportunities: | Impact |
| Emerging therapeutic areas expansion beyond oncology | Broadens the application of RWE across neurology, cardiology, and rare diseases, unlocking new market segments. |
| Focus on patient-generated health data integration | Enhances data richness and personalization, driving innovation in treatment and care delivery models. |
| Market Leaders (2024 ) | |
| Market Leaders |
21% market share |
| Top Players |
Collective market share in 2024 is 65% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Countries | India, Brazil, Mexico, and South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Real World Evidence Solutions Market Trends
- The global RWE solutions market is being driven by the growing need for real-time tracking of drug and device performance. Traditional surveillance methods often suffer from delays and limited data, making it harder to catch safety issues early. RWE platforms overcome this by continuously analysing data from sources like EHRs, claims, registries, and wearables.
- Pharmaceutical firms, regulators, and providers increasingly rely on RWE to detect adverse events and assess treatment effectiveness in near real-time. Additionally, these platforms discover off-label usage patterns or unexpected side effects, allowing for quicker interventions and more informed decision-making further driving adoption across the healthcare ecosystem.
- RWE platforms support dynamic research workflows and faster regulatory reviews, especially critical during public health emergencies or in rapidly evolving therapeutic areas.
- Additionally, the ability to access up-to-date clinical data enhances agility in clinical development. As value-based care gains traction, investment in RWE infrastructure and analytics continues to grow robustly.
Real World Evidence Solutions Market Analysis
Learn more about the key segments shaping this market
Based on the component type, the real-world evidence solutions market is segmented into services and data sets The services segment held a significant market share of 58.4% in 2024.
- The RWE solutions market is segmented into services and data sets, with the services segment holding a dominant position. This is primarily due to the increasing complexity of healthcare data and the critical need for expert interpretation and tailored solutions. Services such as data aggregation, advanced analytics, regulatory consulting, and evidence generation are essential for transforming raw real-world data into actionable insights.
- Moreover, the growing reliance of pharmaceutical companies, payers, and healthcare providers on RWE to guide clinical and commercial decisions is stimulating demand for specialized service providers. These experts ensure data quality, regulatory compliance, and relevance making their role indispensable. Additionally, the rise in chronic diseases and personalized medicine has expanded the use of RWE services across therapeutic areas like oncology and cardiology.
- Advanced technologies like AI and machine learning are being integrated into service offerings, enabling predictive modeling, risk stratification, and early signal detection. Furthermore, regulatory bodies such as the FDA and EMA increasingly accept RWE in submissions, boosting demand for consulting services that help navigate approval pathways. Global outsourcing trends also contribute to this growth, as firms seek cost-effective and scalable RWE solutions.
Based on application, the real world evidence solutions market is segmented into drug development and approvals, medical device development and approvals, post-market surveillance, market access and reimbursement/coverage decision-making and clinical and regulatory decision-making. The drug development and approvals segment was worth USD 840.7 million in 2024.
- Drug development and approval segment dominated the market due to increasing complexity, cost, and risk associated with traditional drug development processes. Pharmaceutical companies are turning to RWE to streamline clinical trials, identify target populations, and monitor long-term safety and effectiveness using real-world data from electronic health records, claims, and registries.
- Moreover, regulatory agencies like the FDA and EMA are actively promoting the use of RWE in drug approvals. The FDA’s RWE Framework supports its use in label expansions, post-approval studies, and new indications accelerating its integration into clinical pipelines. This regulatory support is encouraging pharma companies to adopt RWE earlier in the development cycle.
- RWE is especially valuable in areas like rare diseases and oncology, where randomized controlled trials (RCTs) are often impractical due to small patient populations. In such cases, RWE enables the creation of external control arms and supports accelerated approval pathways. Additionally, AI-driven platforms are enhancing RWE’s utility by enabling predictive modeling, patient stratification, and real-time monitoring.
Based on revenue model, the real world evidence solutions market is segmented into pay-per-use (value-based pricing) and subscription model. The pay-per-use segment held a significant market share of 65.3% in 2024.
- Pay-per-use model is a pricing approach where organizations pay only for the specific data, analytics, or services they use, without long-term contracts. It offers flexibility and cost-efficiency, especially for targeted studies or early-stage research.
- As RWE applications expand across drug development, regulatory submissions, and post-market surveillance, organizations prefer scalable solutions that align with the scope of individual projects. This model allows users to pay only for the specific data, analytics, or consulting services they need minimizing financial risk and optimizing resource allocation.
- Moreover, the rise of cloud-based platforms and AI-powered tools has made modular, on-demand access to RWE services more practical. Startups and emerging biopharma firms especially benefit from this model, gaining access to high-quality insights without large upfront investments. This democratization of access is fostering innovation and accelerating development timelines.
Based on deployment model, the real world evidence solutions market is segmented into on-premises and cloud-based model. The cloud-based segment held a significant market share of 86.3% in 2024.
- Cloud-based deployment refers to delivering RWE solutions via remote servers hosted on the internet, allowing users to access data storage, analytics tools, and computing resources on-demand without needing local infrastructure.
- The cloud-based segment dominates the RWE market due to its ability to handle the growing volume and complexity of real-world data (RWD) from sources like EHRs, claims, registries, and wearables. Cloud platforms offer scalable computing power and flexible storage, making them ideal for processing diverse datasets efficiently.
- Moreover, the rapid adoption of AI and machine learning in RWE analytics requires high-performance environments that cloud platforms are uniquely equipped to provide. These technologies enable predictive modeling, cohort analysis, and real-time insights all supported seamlessly in cloud ecosystems.
- Cloud deployment also enhances collaboration among stakeholders such as pharma companies, CROs, and regulators by enabling secure, shared access to data environments. Additionally, modern cloud platforms offer security features like HIPAA and GDPR compliance making them a choice for handling sensitive healthcare data.
Learn more about the key segments shaping this market
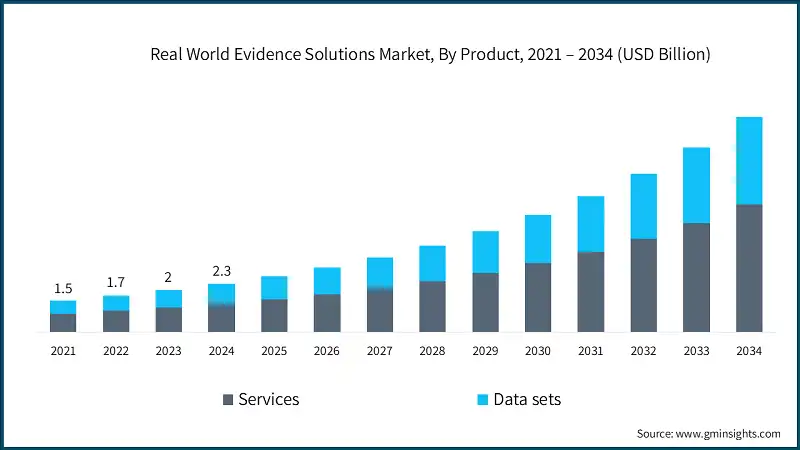
Based on end use, the real world evidence solutions market is categorized into pharmaceutical and medical device companies, healthcare payers, healthcare providers and other end users. Among these, the pharmaceutical and medical device companies segment dominated the market in 2024, accounting for 60.4% of the total market share. This dominance is projected to continue, with the segment expected to reach USD 6.3 billion by 2034.
- Real-world evidence (RWE) is increasingly being adopted by pharmaceutical and medical device companies due to their growing reliance on data-driven strategies for clinical development and regulatory compliance. The increasing complexity of clinical trials and the need for faster, cost-effective pathways have made RWE essential for identifying patient cohorts, simulating trial outcomes, and validating therapeutic efficacy.
- Regulatory agencies like the FDA and EMA are encouraging the use of RWE in drug applications, label expansions, and safety monitoring, prompting life sciences firms to adopt RWE platforms more widely. Medical device companies also use RWE to demonstrate product performance and safety across diverse patient populations, especially in chronic disease and surgical applications.
- Post-market surveillance and pharmacovigilance are key areas where RWE adds value, helping companies detect adverse events and monitor long-term outcomes. Strategic collaborations with RWE solution providers further enhance access to high-quality data, analytics, and regulatory expertise accelerating innovation and improving decision-making.
Looking for region specific data?
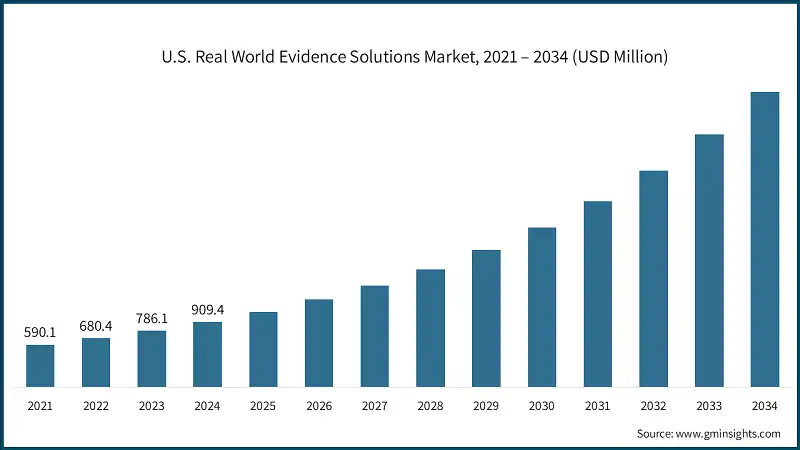
North America Real World Evidence Solutions Market The North America market dominated the market with a market share of 43.2% in 2024. The U.S. real world evidence solutions market was valued at USD 590.1 million and USD 680.4 million in 2021 and 2022, respectively. The market size reached USD 909.4 million in 2024, growing from USD 786.1 million in 2023. Europe market accounted for USD 623.6 million in 2024 and is anticipated to show lucrative growth over the forecast period. Germany real world evidence solutions market is anticipated to witness considerable growth over the analysis period. The Asia Pacific market is anticipated to grow at the CAGR of 16.6% during the analysis timeframe. Japan real world evidence solutions market is predicted to grow significantly over the forecast period. Brazil is experiencing significant growth in the Latin America market due to the high burden of chronic diseases. Saudi Arabia market is poised to witness substantial growth in the Middle East and Africa market during the forecast period. Some of the eminent market participants operating in the Real world evidence solutions industry include: Flatiron Health Inc. is a leading provider of oncology-focused RWE solutions, offering platforms that integrate electronic health records and clinical data to generate actionable insights. Its technology supports cancer research, treatment optimization, and regulatory submissions. By combining real-world data with advanced analytics, Flatiron enables life sciences companies and healthcare providers to improve patient outcomes and accelerate innovation. Strategic partnerships and a deep focus on oncology position Flatiron as a key player in the evolving RWE landscape. ICON plc is a global leader in clinical research and real-world evidence (RWE) solutions, offering end-to-end services across drug development. With deep expertise in oncology and late-phase research, ICON integrates advanced analytics, EHR platforms, and patient-centric technologies to optimize outcomes and accelerate market access. Its proprietary Real World Intelligence™ platform and strategic partnerships empower life sciences companies to generate impactful evidence for regulatory, payer, and provider decisions, positioning ICON as a key innovator in the evolving RWE landscape. Oracle Corporation delivers robust RWE solutions through its Life Sciences division, leveraging over 100 million EHRs and global claims data to support clinical research, market access, and regulatory strategies. Its platforms integrate AI, cloud, and analytics to enable observational studies, patient registries, and safety signal detection. Oracle’s expansive datasets and proprietary tools empower life sciences companies to generate actionable insights, optimize treatment value, and accelerate innovation across the healthcare ecosystem.Europe Real World Evidence Solutions Market
Asia Pacific Real World Evidence Solutions Market
Latin America Real World Evidence Solutions Market
Middle East and Africa Real World Evidence Solutions Market
Real World Evidence Solutions Market Share
Real World Evidence Solutions Market Companies
Neurostimulation Devices Industry News
The real world evidence solutions market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2021 - 2034 for the following segments:
Market, By Component
- Services
- Data collection and integration
- Study design and execution
- Prospective observational studies
- Retrospective database studies
- Site-centric studies
- Registry-based studies
- Hybrid studies
- Regulatory and market access support
- Evidence network
- Other services
- Data sets
- Disparate data sets
- Clinical settings data
- Claims data sets
- Pharmacy data sets
- Patient powered data sets
- Registry-based data sets
- Integrated data sets
- Disparate data sets
Market, By Application
- Drug development and approval
- Oncology
- Cardiovascular disease
- Neurology
- Immunology
- Other therapeutic areas
- Medical device development and approvals
- Post-market surveillance
- Market access and reimbursement/coverage decision-making
- Clinical and regulatory decision-making
Market, By Revenue Model
- Pay-per-use (value-based pricing)
- Subscription
Market, By Deployment Model
- On-premises
- Cloud-based
Market, By End Use
- Pharmaceutical and medical device companies
- Healthcare payers
- Healthcare providers
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Who are the key players in the real-world evidence solutions market?
Key players include Aetion, Inc., Cytel Inc., Flatiron Health Inc., Fortrea Inc., ICON plc, IBM Corporation, IQVIA Holdings Inc., Medidata Solutions, Merative, and Optum, Inc.
Which region leads the real-world evidence solutions market?
North America dominated the market with a 43.2% share in 2024, driven by advanced healthcare infrastructure and increasing adoption of RWE solutions by pharmaceutical companies and regulatory bodies.
What are the upcoming trends in the real-world evidence solutions market?
Key trends include the growing need for real-time tracking of drug and device performance, adoption of RWE platforms for dynamic research workflows, and faster regulatory reviews, especially during public health emergencies or in rapidly evolving therapeutic areas.
What was the valuation of the cloud-based deployment segment?
The cloud-based deployment segment held 86.3% market share in 2024.
How much revenue did the drug development and approvals segment generate?
The drug development and approvals segment generated USD 840.7 million in 2024.
What was the valuation of the pay-per-use revenue model segment?
The pay-per-use revenue model held 65.3% market share and generated significant revenue in 2024.
What is the projected value of the real-world evidence solutions market by 2034?
The market is expected to reach USD 10.2 billion by 2034, fueled by increasing adoption of RWE solutions for reimbursement decision-making and advancements in data analytics platforms.
What is the projected size of the real-world evidence solutions market in 2025?
The market is expected to reach USD 2.6 billion in 2025.
Real World Evidence Solutions Market Scope
Related Reports


