Summary
Table of Content

North America Pancreatic Cancer Diagnostic Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
North America Pancreatic Cancer Diagnostic Market Size
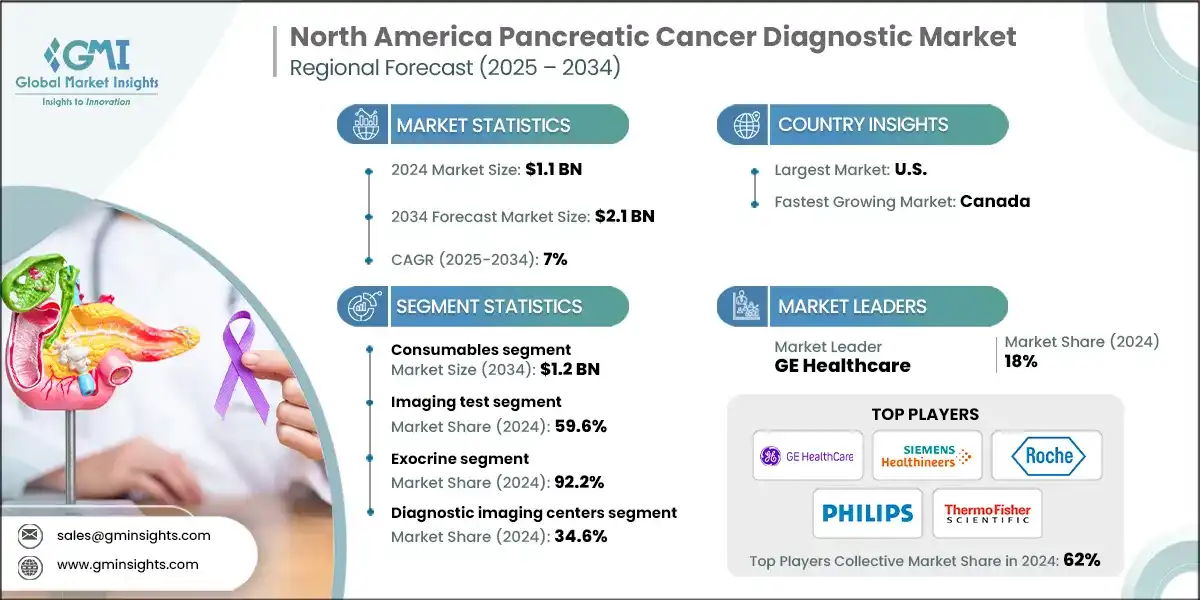
The North America pancreatic cancer diagnostic market was estimated at USD 1.1 billion in 2024. The market is expected to be valued at USD 2.1 billion in 2034, growing at a CAGR of 7%, according to the latest report published by Global Market Insights Inc. The market is growing due to the rising prevalence of pancreatic cancer in North America, advancements in diagnostic technologies, increased awareness through screening initiatives, and higher healthcare spending in the region.
To get key market trends
Pancreatic cancer diagnostics involve medical tools and technologies designed to detect and monitor pancreatic cancer, often identified at advanced stages because of its subtle early symptoms. In North America, the pancreatic cancer diagnostic market is evolving rapidly with the adoption of innovative solutions such as high-resolution imaging systems, biopsy instruments, liquid biopsy kits, and next-generation sequencing platforms. These advanced tools are commonly used in hospitals, laboratories, and research centers to improve diagnostic accuracy and support earlier interventions.
Major companies in the market include F. Hoffmann-La Roche, Thermo Fisher Scientific, GE Healthcare, Siemens Healthineers, and Koninklijke Philips. With a strong focus on personalized medicine and ongoing investments in research and development, North America is at the forefront of pancreatic cancer diagnostics, creating opportunities to improve patient outcomes and transform the approach to pancreatic cancer care.
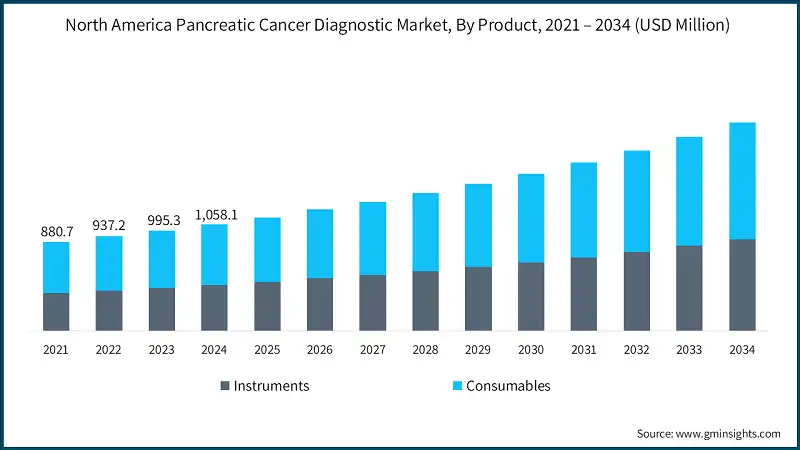
The market grew from USD 880.7 million in 2021 to USD 995.3 million in 2023. This growth has been driven by growing awareness, early screening programs, and advancements in diagnostic tools. Precision diagnostics are becoming more common, with tools such as endoscopic ultrasound, genetic testing panels, and radiomics-based imaging analysis being widely adopted. These innovations are helping clinicians detect tumors earlier and create more personalized treatment plans.
Additionally, the use of cloud-based data platforms and AI-powered diagnostic systems is making clinical decision-making more efficient and improving the reliability of diagnoses. The region’s dedication to research-focused healthcare, better patient outcomes, and partnerships between healthcare providers and technology developers continues to fuel market growth.
The increasing prevalence of pancreatic cancer in North America is a major factor driving market growth. For example, the National Cancer Institute reported that approximately 107,988 people in the U.S. were living with pancreatic cancer in 2022. Furthermore, an estimated 67,440 new cases are expected to be diagnosed by 2025. This rising trend has created a growing demand for better diagnostic technologies, earlier detection methods, and more effective treatment options. As the number of affected individuals increases, healthcare providers and industry leaders are stepping up their efforts and investments to address this urgent medical challenge.
Advancements in diagnostic technologies are also transforming pancreatic cancer care across North America. These innovations are improving the accuracy, speed, and reliability of cancer detection, allowing for earlier and more precise diagnoses. For instance, GE Healthcare’s SIGNA Champion 1.5T MRI Scanner is a notable advancement in imaging technology. With AI-powered features such as AIR Recon DL and Sonic DL, this system provides high-resolution images in less time, boosting diagnostic confidence and improving patient outcomes. These technological advancements are encouraging healthcare providers to adopt more advanced diagnostic tools, leading to better clinical decisions and more personalized treatment strategies for pancreatic cancer management.
Pancreatic cancer diagnostics involve a variety of methods and technologies designed to detect the disease at different stages. These include imaging techniques, biomarker tests, molecular diagnostics, and biopsy procedures, all of which work together to confirm the presence of cancer and guide treatment decisions.
North America Pancreatic Cancer Diagnostic Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 1.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 7% |
| Market Size in 2034 | USD 2.1 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of pancreatic cancer in North America | Drives demand for early, accurate, and non-invasive diagnostic solutions. Rising incidence rates have led to increased screening and diagnostic volumes, especially in high-risk populations. |
| Advancements in diagnostic technologies | Fuels adoption of precision tools such as liquid biopsies, molecular profiling, and AI-integrated imaging systems. These innovations improve early-stage detection and diagnostic accuracy. |
| Increased awareness and screening programs | Promotes routine checkups and early detection, especially among genetically predisposed individuals and older adults. Public health campaigns and funding initiatives are boosting screening uptake. |
| Rising healthcare expenditure in North America | Encourages investment in advanced diagnostic infrastructure and technologies, supporting innovation and improved patient outcomes. Enables broader access to high-end diagnostic services. |
| Pitfalls & Challenges | Impact |
| Stringent regulatory scenario | Increases operational complexity for manufacturers. Compliance with sterility, labeling, and traceability standards can slow product development and market entry. |
| High cost of diagnostics and treatment | Restricts access to advanced diagnostics in low-income countries, despite clinical need. Hampers widespread adoption and market penetration. |
| Opportunities: | Impact |
| Growing adoption of AI in diagnostics | Enhances diagnostic precision and efficiency. AI-powered imaging and biomarker analysis tools are increasing diagnostic throughput and enabling personalized care, especially for high-risk groups. |
| Market Leaders (2024) | |
| Market Leaders |
18% market share |
| Top Players |
Collective Market Share is 62% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | U.S. |
| Fastest Growing Market | Canada |
| Future outlook |
|
What are the growth opportunities in this market?
North America Pancreatic Cancer Diagnostic Market Trends
- The growing awareness and screening programs across North America are playing a crucial role in boosting the demand for early pancreatic cancer diagnostics. Public health campaigns, advocacy efforts, and educational outreach are encouraging individuals at high risk, such as those with a family history or genetic predisposition, to prioritize regular screenings and risk assessments.
- For example, organizations such as the Pancreatic Cancer Action Network (PanCAN) and the Centers for Disease Control and Prevention (CDC) have introduced nationwide awareness campaigns and screening guidelines to promote early detection. These efforts are particularly focused on older adults and underserved communities, where late-stage diagnoses are more common. Thus, more people are using diagnostic services, and screening programs are reaching a wider audience within healthcare systems.
- Screening programs typically include imaging tests, blood-based biomarker analysis, and genetic testing to detect early signs of pancreatic abnormalities. This has created a steady demand for diagnostic tools like endoscopic ultrasound (EUS), MRI, and molecular profiling kits, which are used in both primary care and specialized oncology settings.
- In addition, advancements in AI-powered risk assessment tools and non-invasive screening technologies are making these programs even more effective. For instance, AI-integrated imaging platforms and liquid biopsy tests are helping detect pancreatic cancer earlier and more accurately, especially in individuals showing no symptoms. These innovations are driving the growth of the pancreatic cancer diagnostics market by supporting proactive screening and early intervention strategies.
North America Pancreatic Cancer Diagnostic Market Analysis
Learn more about the key segments shaping this market
Based on the product, the pancreatic cancer diagnostic market is segmented into instruments and consumables. The consumables segment led this market in 2024, accounting for the highest market share because of its high demand for reagents and assay kits and the growing adoption of liquid biopsy and molecular testing techniques. The consumables segment was valued at USD 602.1 million in 2024 and is projected to reach USD 1.2 billion by 2034, growing at a CAGR of 6.8%. This growth is due to the shift toward early detection and precision oncology and boosting demand for repeat-use consumables. In comparison, the North America pancreatic cancer diagnostic market from instruments segment, valued at USD 456 million in 2024, is expected to grow to USD 909.7 million by 2034, with a slightly higher CAGR of 7.2%, supported by the integration of high-performance imaging modalities and precision diagnostic tools.
- The growing emphasis on awareness and screening programs across North America is significantly driving the demand for early pancreatic cancer diagnostics. Public health initiatives, advocacy campaigns, and educational outreach encourage high-risk individuals, such as those with a family history or genetic predisposition, to undergo regular screening and risk assessment.
- For instance, organizations such as the Pancreatic Cancer Action Network (PanCAN) and the Centers for Disease Control and Prevention (CDC) have launched nationwide awareness campaigns and screening guidelines aimed at early detection. These efforts particularly focus on older adults and underserved populations, where late-stage diagnoses are more common. Such initiatives are increasing the uptake of diagnostic services and expanding the reach of screening programs across healthcare systems.
- Screening programs often involve imaging tests, blood-based biomarker analysis, and genetic testing to identify early signs of pancreatic abnormalities. This drives consistent demand for diagnostic tools such as endoscopic ultrasound (EUS), MRI, and molecular profiling kits in both primary care and specialized oncology settings.
- Additionally, advancements in AI-powered risk assessment tools and non-invasive screening technologies are enhancing the effectiveness of these programs. For example, AI-integrated imaging platforms and liquid biopsy tests enable earlier and more accurate detection, particularly in asymptomatic individuals. These trends contribute to the sustained growth of the pancreatic cancer diagnostics market through proactive screening and early intervention strategies.
Based on the test type, the North America pancreatic cancer diagnostic market is segmented into imaging test, biopsy, blood test, and other test type. The imaging test segment accounted for the highest market share of 59.6% in 2024.
- The imaging test segment includes diagnostic tools such as computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), and positron emission tomography (PET). These technologies are crucial for visualizing pancreatic tumors, tracking disease progression, and helping doctors make decisions about biopsies or surgeries.
- In North America, the imaging test segment leads the pancreatic cancer diagnostics market because it is widely accessible and seamlessly integrated into everyday clinical practices. These tests are invaluable as they allow non-invasive imaging of pancreatic structures, which is essential for early detection, staging, and planning treatments.
- Additionally, hospitals and cancer centers across North America are equipped with advanced imaging systems, enabling quick and accurate tumor localization. The growing use of imaging to monitor treatment responses and guide surgeries highlights its critical role in managing pancreatic cancer.
- GE HealthCare’s Discovery MI Gen 2 PET/CT system is a prime example of innovation in this field. It uses digital detectors and AI-powered reconstruction algorithms to provide clearer images and faster scan times. This system supports theragnostic, which combines diagnostic imaging with therapy planning, and is increasingly being used in oncology centers for staging and monitoring pancreatic cancer.
- Further, the imaging test segment will remain a cornerstone of pancreatic cancer diagnostics. It offers non-invasive ways to detect the disease early and create personalized treatment plans. With ongoing advancements in imaging technology and increasing demand from clinicians, this segment is expected to see steady growth across North America.
Based on the cancer type, the North America pancreatic cancer diagnostic market is segmented into exocrine and endocrine. The exocrine segment accounted for the highest market share of 92.2% in 2024.
- The pancreatic cancer diagnostics market in North America is growing rapidly, driven by the rising number of exocrine pancreatic cancer cases. These include adenocarcinoma, colloid carcinoma, adenosquamous carcinoma, and squamous cell carcinoma (SCC). Each of these subtypes requires precise diagnostic methods to ensure accurate classification and effective treatment planning.
- Accurate diagnosis is especially important when distinguishing primary pancreatic SCC from squamous tumors that have spread from other organs. For example, the Skin Cancer Foundation reports that around 1.8 million cases of squamous cell carcinoma are diagnosed annually in the U.S., mostly as skin cancers. This highlights the need for careful differential diagnosis in pancreatic cases.
- Further, diagnostic evaluations start with advanced imaging techniques like CT and MRI, followed by confirmation through histopathology. Immunohistochemistry (IHC) markers such as p63, CK5/6, and cytokeratin are commonly used to confirm squamous differentiation, ensuring precise diagnoses.
- Moreover, the market for exocrine pancreatic cancer diagnostics is also expanding due to rising incidence rates, particularly among older populations. The use of artificial intelligence in imaging and pathology, along with advancements in liquid biopsy techniques, is improving diagnostic accuracy and enabling earlier interventions. These developments are contributing to better patient outcomes across North America.
Learn more about the key segments shaping this market
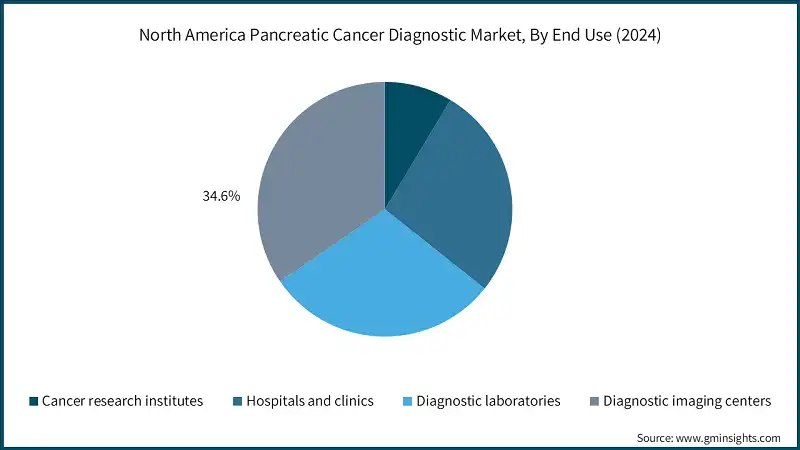
Based on end use, the North America pancreatic cancer diagnostic market is segmented into cancer research institutes, hospitals and clinics, diagnostic laboratories, and diagnostic imaging centers. In 2024, diagnostic imaging centers held the largest share of 34.6%.
- Diagnostic imaging centers play an important role in helping to detect and stage pancreatic cancer early by providing non-invasive ways to see internal organs and tissues. Using advanced tools such as CT scans, MRI, PET scans, and endoscopic ultrasound (EUS), these centers help doctors find tumors, track the disease progress, and guide biopsy procedures. For exocrine pancreatic tumors, imaging is often the first step in confirming a diagnosis and planning treatment.
- In North America, diagnostic imaging benefits from a strong infrastructure, supported by widespread public awareness, active screening programs, and reimbursement policies that make access easier. With pancreatic cancer cases increasing in the U.S. and Canada, the need for accurate and timely imaging has grown, making these centers a vital part of cancer care.
- Additionally, diagnostic imaging centers are critical for early diagnosis and creating personalized treatment plans. As the field continues to advance with technologies such as artificial intelligence and molecular imaging, these centers are expected to stay at the forefront of innovation, helping to improve outcomes for patients with pancreatic cancer.
Looking for region specific data?
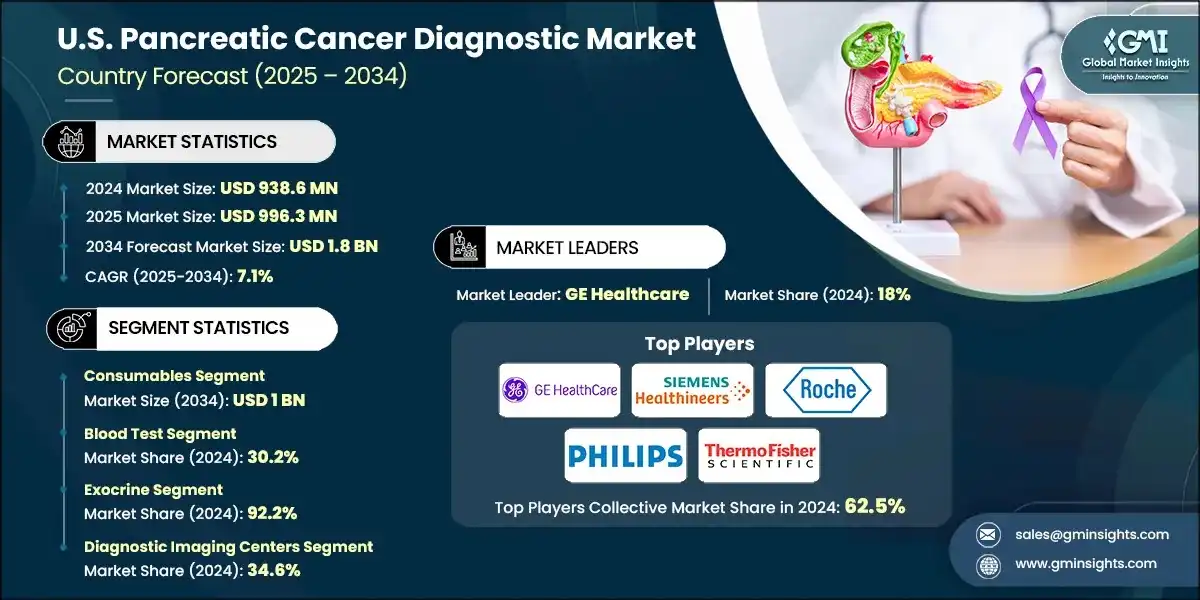
The U.S. dominated the North America pancreatic cancer diagnostic market with a market share of 88.7% in 2024. The market is driven by the rising incidence of pancreatic cancer and the presence of robust healthcare infrastructure. Advancements in imaging technology and a growing focus on early and accurate diagnosis also support market growth.
- The U.S. pancreatic cancer diagnostic market was valued at USD 779.9 million and USD 830.3 million in 2021 and 2022, respectively. In 2024, the market size reached USD 938.6 million from USD 882.4 million in 2023.
- The increasing number of pancreatic cancer cases in the U.S. is playing a major role in driving market growth.
- For instance, as reported by the World Cancer Research Fund, around 60,127 new cases of pancreatic cancer were identified in the U.S. in 2022, with 31,598 cases among men and 28,529 among women. By 2025, this number is expected to rise to 67,440 new diagnoses, with over 51,980 deaths anticipated. These alarming statistics emphasize the urgent need for earlier detection and better diagnostic tools.
- With the growing impact of pancreatic cancer, there is a rising demand for advanced diagnostic tools that can enable early detection, accurate staging, and personalized treatment plans. This demand is driving the adoption of innovative imaging systems, molecular diagnostics, and AI-powered platforms across the U.S.
- Additionally, the U.S. is establishing itself as a leader in pancreatic cancer diagnostic technologies. This progress is supported by a strong healthcare system, widespread use of advanced imaging technologies, and close collaboration between public institutions and private companies. Together, these factors are positioning the U.S. as a hub of innovation and growth in pancreatic cancer diagnostics.
Canada pancreatic cancer diagnostic market accounted for USD 119.5 million in 2024 and is anticipated to show lucrative growth over the forecast period.
- The rising prevalence of pancreatic cancer in the country, combined with increasing awareness and screening initiatives, is expected to drive market growth.
- For example, the Canadian Cancer Society estimates that in 2024, approximately 7,100 Canadians will be diagnosed with pancreatic cancer, including about 3,800 men and 3,300 women. Sadly, around 6,100 Canadians are expected to lose their lives to the disease, making it the third leading cause of cancer-related deaths in the country. These numbers highlight the critical need for better diagnostic tools and earlier interventions.
- Canada is taking a proactive approach to cancer care, supported by government funding and collaborative research efforts. These initiatives are helping to tackle the challenges of late-stage diagnoses and improve outcomes for patients across the country.
North America Pancreatic Cancer Diagnostic Market Share
- The top five players, F. Hoffmann-La Roche, Thermo Fisher Scientific, GE Healthcare, Siemens Healthineers, and Koninklijke Philips, collectively hold 62% of the market share in the North American pancreatic cancer diagnostic market. These companies continue to strengthen their positions through innovation, regulatory compliance, and strategic collaborations. They are making significant investments in technology by introducing next-generation biopsy tools that enhance precision, real-time imaging, and minimally invasive procedures.
- GE Healthcare plays a pivotal role by offering advanced imaging systems such as the SIGNA Premier MRI and Discovery MI Gen 2 PET/CT. These platforms combine high-resolution imaging with AI-powered reconstruction and theranostic capabilities, supporting both diagnosis and treatment planning. Their integration into oncology workflows across North America enhances early detection and staging of pancreatic cancer.
- Manufacturers across the region are adopting value-based pricing strategies to improve accessibility in cost-sensitive markets while also launching AI-assisted platforms for pancreatic cancer screening. These platforms include features such as real-time molecular profiling, automated lesion detection, and integrated reporting tools, which expand access to precision diagnostics in outpatient and decentralized care settings.
- Emerging trends in the North American pancreatic cancer diagnostics market include the development of minimally invasive liquid biopsy tools, AI-powered imaging systems, and workflow-integrated platforms designed for early detection and personalized oncology. Technologies such as circulating tumor DNA (ctDNA) assays, contrast-enhanced endoscopic ultrasound, and radiomics-based AI models are significantly improving diagnostic precision, accessibility, and patient outcomes.
North America Pancreatic Cancer Diagnostic Market Companies
Few of the prominent players operating in the North America pancreatic cancer diagnostic industry include:
- Abbott Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Canon
- Danaher Corporation
- F. Hoffmann-La Roche
- GE Healthcare
- Illumina
- Koninklijke Philips
- Myriad Genetics
- QIAGEN
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific
- F. Hoffmann-La Roche
F. Hoffmann-La Roche has a strong global workforce of approximately 103,249 employees, which enables the company to drive innovation and deliver high-quality solutions.
GE HealthCare holds a significant share in the pancreatic cancer diagnostic market through its comprehensive product portfolio. GE HealthCare emphasizes R&D and product development offerings with advanced imaging capabilities, ergonomic designs, and AI-powered diagnostic tools.
Siemens Healthineers holds a significant share in the North America pancreatic cancer diagnostic market through its comprehensive product portfolio. It includes the FDA-cleared NAEOTOM Alpha Photon-Counting CT Scanner and Atellica CI Analyzer, among others.
North America Pancreatic Cancer Diagnostic Industry News:
- In July 2024, Becton, Dickinson and Company (BD) partnered with Quest Diagnostics to co-develop and commercialize flow cytometry-based companion diagnostics (CDx) for cancer, including pancreatic cancer. This collaboration combines BD’s expertise in flow cytometry with Quest’s strengths in biomarker validation and clinical trial support. The partnership aims to deliver end-to-end CDx solutions, from development to FDA-approved distribution, and advance personalized oncology care in North America.
- In July 2025, Philips received FDA 510(k) clearance for its SmartSpeed Precise Dual AI software, a breakthrough in AI-powered MRI technology. The solution enables up to three times faster scans and 80% sharper images with a single click. It is now approved for use across Philips’ entire 1.5T and 3.0T MRI portfolio. This advancement enhances workflow efficiency and diagnostic precision, reinforcing Philips’ commitment to scalable, AI-integrated imaging solutions for high-impact clinical applications, including pancreatic cancer diagnostics.
The North America pancreatic cancer diagnostic market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Product
- Instruments
- Consumables
Market, By Test Type
- Imaging test
- CT scan
- MRI
- Ultrasound
- PET
- Other imaging tests
- Biopsy
- Blood test
- Liver function tests
- Tumor markers
- Other blood tests
- Other test types
Market, By Cancer Type
- Exocrine
- Adenocarcinoma
- Colloid carcinoma
- Adenosquamous carcinoma
- Squamous cell carcinoma
- Endocrine
Market, By End Use
- Cancer research institutes
- Hospitals and clinics
- Diagnostic laboratories
- Diagnostic imaging centers
The above information is provided for the following countries:
- U.S.
- Canada
Frequently Asked Question(FAQ) :
Which end use segment dominated the market in 2024?
Diagnostic imaging centers dominated the market in 2024, holding a 34.6% share, due to their widespread adoption for early and accurate diagnosis.
Which country leads the North America pancreatic cancer diagnostic market?
The United States led the market with an 88.7% share in 2024, driven by a high incidence of pancreatic cancer, robust healthcare infrastructure, and advancements in imaging technology.
Who are the key players in the North America pancreatic cancer diagnostic market?
Key players include Abbott Laboratories, Agilent Technologies, Becton, Dickinson and Company, Boston Scientific Corporation, Canon, Danaher Corporation, F. Hoffmann-La Roche, GE Healthcare, Illumina, Koninklijke Philips, and Myriad Genetics.
Which test type held the largest market share in 2024?
The imaging test segment held the largest market share of 59.6% in 2024, driven by advancements in imaging technologies and their critical role in early detection.
What was the valuation of the instruments segment in 2024?
The instruments segment was valued at USD 456 million in 2024, supported by the integration of high-performance imaging modalities and precision diagnostic tools.
What is the projected size of the instruments segment by 2034?
The instruments segment is expected to grow to USD 909.7 million by 2034, with a CAGR of 7.2%.
What is the projected size of the consumables segment by 2034?
The consumables segment is projected to reach USD 1.2 billion by 2034, growing at a CAGR of 6.8%, driven by the shift toward early detection and increased demand for repeat-use consumables.
What was the valuation of the consumables segment in 2024?
The consumables segment was valued at USD 602.1 million in 2024, leading the market due to high demand for reagents, assay kits, and the adoption of liquid biopsy and molecular testing techniques.
What is the market size of the North America pancreatic cancer diagnostic in 2024?
The market size was USD 1.1 billion in 2024, with a CAGR of 7% expected through 2034, driven by the rising prevalence of pancreatic cancer, advancements in diagnostic technologies, increased awareness through screening initiatives, and higher healthcare spending.
What is the projected value of the North America pancreatic cancer diagnostic market by 2034?
The market is expected to reach USD 2.1 billion by 2034, supported by technological advancements, early detection initiatives, and growing demand for precision oncology solutions.
North America Pancreatic Cancer Diagnostic Market Scope
Related Reports


