Summary
Table of Content

North America Epilepsy Treatment Drugs Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
North America Epilepsy Treatment Drugs Market Size
The North America epilepsy treatment drugs market was valued at USD 3.42 billion in 2024 and is projected to grow from USD 3.55 billion in 2025 to USD 5.25 billion by 2034, expanding at a CAGR of 4.4% according to the latest report published by Global Market Insights Inc.
To get key market trends
The growth of the North America epilepsy treatment drugs market is driven by several factors, including rising prevalence of epilepsy, increasing aging population in North America, increasing demand for novel treatment for epilepsy, and supportive government policies and funding. Key players in this market include, Pfizer, Novartis AG, GSK (GlaxoSmithKline), SK Biopharmaceuticals.
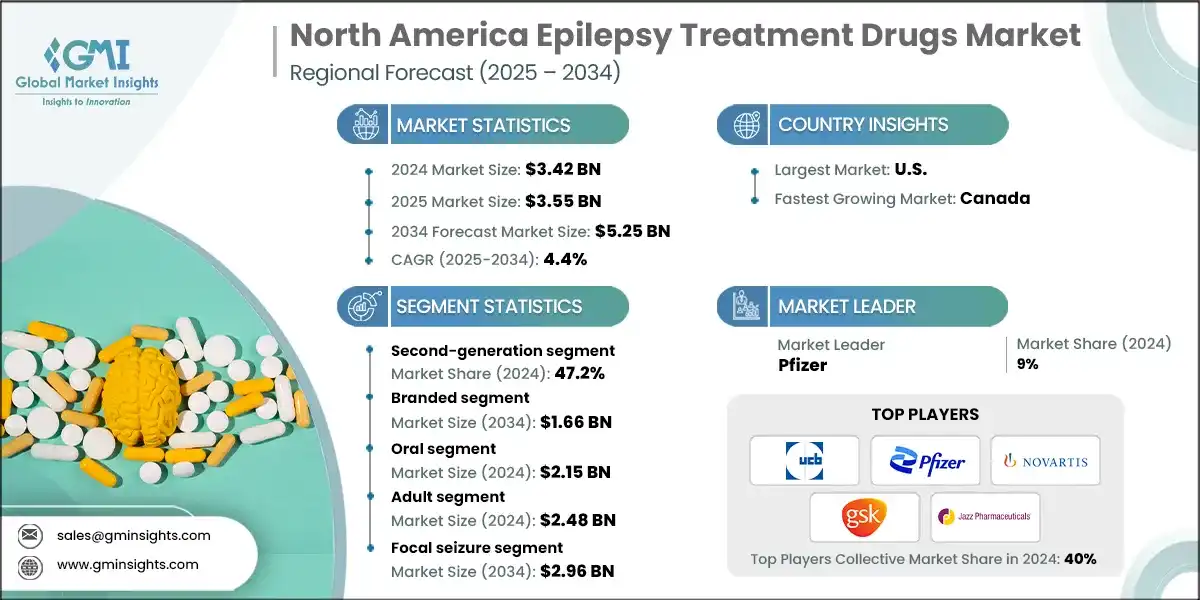
The epilepsy treatment drugs market grew from USD 3.15 billion in 2021 to USD 3.30 billion in 2023, driven primarily by the rising prevalence of epilepsy. In the U.S. alone, nearly 3 million adults live with epilepsy, a condition more common among individuals with neurological or genetic disorders. This widespread prevalence creates a strong need for antiepileptic drugs, encouraging pharmaceutical companies to invest in research and development. As more patients are diagnosed, the demand for effective treatment options continues to rise, prompting innovation and expansion of drug portfolios to address different types of epilepsy and patient needs. Additionally, increased awareness and improved diagnostic capabilities enable earlier detection and treatment, further fueling market growth.
The rapidly growing aging population in the U.S. is a significant factor driving demand for epilepsy treatment drugs. Individuals aged 65 and older are more vulnerable to neurological disorders, including epilepsy, due to age-related changes in brain function and higher prevalence of comorbid conditions. For instance, according to the Population Reference Bureau, the number of people in this age group is projected to rise from 58 million in 2022 to 82 million by 2050, a 42% increase.
Their share of the total population will also grow from 17% to 23% during the same period. As the proportion of older adults increases, so does the need for effective and safe epilepsy medications becomes important to manage seizures and improve quality of life, thereby fueling growth in the epilepsy treatment drugs market.
Epilepsy treatment drugs also known as anti-seizure or anticonvulsant medications are pharmaceutical agent used to control and prevent seizures in individuals diagnosed with epilepsy. These drugs work by stabilizing electrical activity in the brain, limiting the spread of abnormal signal that cause seizures. They are considered the first line and most common form of treatment for epilepsy.
North America Epilepsy Treatment Drugs Market Trends
- Supportive government policies and sustained funding are key drivers of growth in the epilepsy treatment drugs market, as they provide a strong foundation for research, innovation, and accessibility. Agencies such as the CDC, NIH, and Epilepsy Centers of Excellence allocate millions annually to programs focused on seizure recognition, training, surveillance systems, and drug development initiatives.
- For example, in 2025, the Epilepsy Foundation allocated USD 12.5 million to the CDC’s epilepsy program, USD 26.8 million to the Veterans Affairs Epilepsy Centers of Excellence, and USD 15 million to establish a Pediatric-Onset Epilepsies Network. These investments accelerate the discovery of novel antiepileptic drugs and strengthen healthcare infrastructure.
- Such efforts ensure epilepsy remains a public health priority, encouraging pharmaceutical companies to develop advanced therapies with improved efficacy and fewer side effects. Increased funding also drives clinical trials, regulatory approvals, and public-private partnerships, boosting the availability and adoption of new treatment options.
- Therefore, supportive government policies and sustained funding play an important role in expanding the epilepsy treatment drugs market by fostering innovation, improving access to care, and addressing unmet medical needs.
North America Epilepsy Treatment Drugs Market Analysis
Learn more about the key segments shaping this market
Based on drug class, the market is segmented into first-generation, second-generation, and third generation. The second-generation segment dominated the market with the largest revenue share of 47.2% in 2024 and is expected to grow at a CAGR of 4.4% over the forecast period.
- Second-generation antiepileptic drugs dominate the epilepsy treatment market due to their improved safety and efficacy compared to first-generation drugs. Older drugs often cause significant side effects and complex drug interactions, whereas second-generation therapies provide better seizure control with fewer adverse reactions.
- Their suitability for long-term use, particularly among vulnerable groups such as children and the elderly, has strengthened their adoption. Additionally, their ability to treat a broader range of seizure types with greater precision makes them the preferred choice for neurologists and healthcare providers.
- Further, second-generation drugs have become the market standard, meeting the evolving needs of both patients and clinicians. Their superior performance, reduced side effects, and adaptability for personalized treatment approaches position them as essential in modern epilepsy management.
Based on type, the epilepsy treatment drugs market is bifurcated into branded and generics. The branded segment held a revenue of USD 1.05 billion in 2024 and is expected to reach USD 1.66 billion by 2034.
- Leading pharmaceutical companies are increasingly investing in branded epilepsy drugs that offer better tolerability, fewer side effects, and improved effectiveness. This trend is driving growth in the branded drug segment as patients and healthcare providers seek safer and more reliable treatment options.
- These newer medications are designed to overcome the limitations of older drugs, making them more suitable for long-term use and better aligned with individuals patient needs. For example, Xcopri (cenobamate), developed by SK Life Science and launched in 2020, is indicated for the treatment of partial-onset seizures in adults. Its dual mechanism of action, enhancing GABAergic inhibition and reducing sodium channel activity, provides strong seizure control, particularly for patients with treatment-resistant epilepsy.
- Moreover, the growing focus on branded epilepsy treatments is fueled by both innovation and necessity. As companies continue to develop advanced therapies that deliver better outcomes for patients with challenging conditions, the branded drug segment is expected to expand significantly.
Based on route of administration, the North America epilepsy treatment drugs market is segmented into oral, nasal, injectable, and rectal. The oral segment dominated the market with the largest revenue of USD 2.15 billion in 2024 and is expected to grow at a CAGR of 4.7% over the forecast period.
- Oral epilepsy treatment drugs refer to medications administered by mouth, typically as tablets, capsules, or liquids. This route is the most common and preferred method for both patients and healthcare providers because it is convenient, easy to use, and non-invasive.
- Most antiepileptic drugs (AEDs), including first-line and newer-generation treatments, are available in oral form. The simplicity of oral administration improves patient adherence to treatment regimens, which enhances outcomes and drives demand.
- Additionally, oral formulations are often cost-effective and can be self-administered, reducing the need for frequent clinical visits or specialized care. This makes them practical for long-term management of epilepsy.
- Therefore, the oral segment continues to lead the epilepsy treatment market, combining effectiveness with ease of use, affordability, and patient comfort.
Based on age group, the North America epilepsy treatment drugs market is bifurcated into pediatric and adult. The adult segment dominated the market with the largest revenue of USD 2.48 billion in 2024 and is expected to grow at a CAGR of 4.3% over the forecast period.
- The adult segment dominates the epilepsy treatment drugs market due to the high prevalence of epilepsy among adults compared to children. Adults are more likely to develop epilepsy as a result of factors such as head injuries, strokes, brain tumors, and neurodegenerative diseases, which occur more frequently in older age groups. Thus, the demand for antiepileptic drugs is significantly higher in this population.
- Pharmaceutical companies often prioritize adult patients when launching new drugs, as this group represents a larger and more stable market. The availability of a wide range of oral and branded medications tailored to adult needs further supports the segment’s growth.
- Moreover, the adult segment continues to lead the epilepsy treatment drugs market, driven by higher diagnosis rates, greater access to advanced therapies, and its substantial patient base.
Based on seizure type, the North America epilepsy treatment drugs market is segmented into focal seizure, generalized seizure, and combined seizure. The focal seizure segment held a market revenue of USD 1.93 billion in 2024 and is expected to reach USD 2.96 billion by 2034.
- Focal seizures originate from a specific area of the brain and typically affect only one part of the body. These seizures are more common and easier to diagnose compared to generalized seizures.
- Their high prevalence, especially among adults, has created strong demand for targeted medications that effectively manage these seizure types. This has led to increased prescriptions and the development of specialized antiepileptic drugs.
- For instance, research indicates that the prevalence of focal onset seizures among children aged 0–4 years ranges from 0.15% in Canada to 0.61% in the U.S.
- Advancements in diagnostic tools, such as AI-powered analytics, enhanced EEGs, and genetic testing, have improved early identification of focal epilepsy. These advancements enable more accurate and personalized treatment plans. Pharmaceutical companies are responding by focusing research and development efforts on therapies that offer better seizure control with fewer side effects for focal seizure patients.
- Furthermore, the focal seizure segment continues to lead the epilepsy treatment drugs market, driven by its high prevalence, improved diagnostic capabilities, and availability of specialized therapies.
Learn more about the key segments shaping this market
Based on distribution channel, the North America epilepsy treatment drugs market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment held market revenue of USD 2.23 billion in 2024 and is expected to grow at a CAGR of 4.4% over the forecast period.
- Hospitals serve as the primary point of care for patients experiencing seizures, making hospital pharmacies a critical component of epilepsy treatment. These pharmacies are directly connected to neurologists and epilepsy specialists, ensuring accurate prescribing and dispensing of antiepileptic drugs (AEDs).
- Additionally, hospital pharmacies are equipped to manage prescriptions for newer-generation AEDs and injectable formulations, which are often unavailable in retail or online settings. This capability, combined with the trust and convenience of receiving medications directly from a healthcare facility, promotes higher patient adherence and better treatment outcomes.
- Moreover, hospital pharmacies dominate the epilepsy treatment drugs market due to their close integration with specialized care, access to advanced medications, and their role in initiating and managing comprehensive treatment plans.
Looking for region specific data?
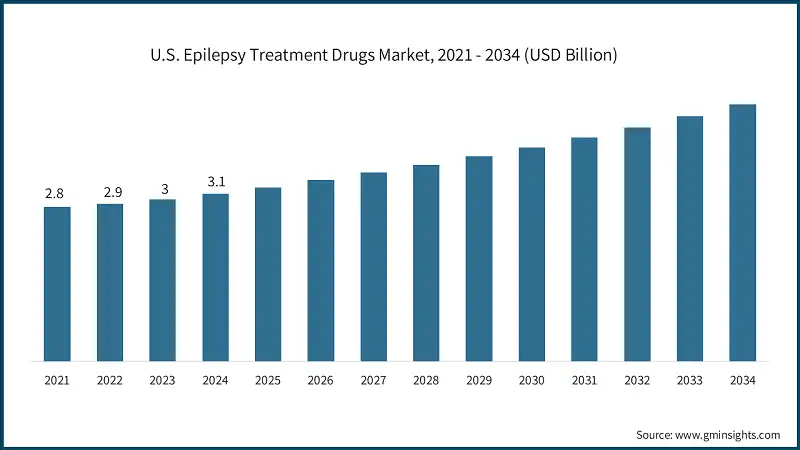
U.S. dominated the North America epilepsy treatment drugs market, with the highest market share of 90.1% in 2024. The U.S. epilepsy treatment drugs market was valued at USD 2.8 billion and USD 2.9 billion in 2021 and 2022, respectively. In 2024, the market size reached USD 3.1 billion from USD 3 billion in 2023.
- The growing aging population in the U.S. is a key driver of the epilepsy treatment drugs market. As individuals age, the risk of developing neurological disorders such as epilepsy increases due to factors such as stroke, brain tumors, and neurodegenerative diseases. This demographic shift is creating a larger patient pool that requires long-term epilepsy management, thereby fueling market growth.
- For example, the number of people aged 65 and older is projected to increase from 58 million in 2022 to 82 million by 2050, a 47% surge. This significant expansion highlights the urgent need for accessible and effective epilepsy treatments.
- Additionally, supportive government policies and funding initiatives are playing a crucial role in strengthening the market. These programs ensure that more individuals have access to necessary treatments, further accelerating growth.
- Thus, the combination of an increasing aging population and favorable government support is driving the expansion of the epilepsy treatment drugs market in the U.S.
Canada epilepsy treatment drugs market accounted for USD 339.9 million in 2024 and is anticipated to show lucrative growth over the forecast period.
- The rising prevalence of epilepsy in Canada is a significant driver of growth in the epilepsy treatment drugs market. As more individuals across various age groups are diagnosed with epilepsy, the demand for effective treatment options continues to increase.
- For instance, data on epilepsy prevalence in Canada reveals that 13% of affected individuals are children and youth aged 1–19 years, 63% are adults, aged 20–64 years, and 24% are older adults aged 65 and above. This distribution demonstrates that epilepsy impacts all age groups, creating a need for a diverse range of treatment solutions tailored to different demographics.
- Thus, the growing prevalence of epilepsy across all age groups in Canada is fueling the expansion of the epilepsy treatment drugs market.
North America Epilepsy Treatment Drugs Market Share
- In North America's epilepsy treatment drugs market, leading pharmaceutical companies such as UCB, Pfizer, Novartis AG, GSK (GlaxoSmithKline), and Jazz Pharmaceuticals are strengthening their presence through diverse product portfolios, robust regulatory compliance, and continuous innovation. Collectively, these top five players account for about 40% of the market share. Their growth is further fueled by strategic collaborations with research organizations, healthcare providers, and global distributors, enabling broader access to advanced treatment options.
- New players aiming to enter the epilepsy treatment drugs market must adopt strategic approaches to compete with established pharmaceutical giants. Developing novel formulations, targeting niche conditions such as drug-resistant or pediatric epilepsy, and exploring precision medicine can help differentiate their offerings.
- Strategic partnerships with research institutions, biotech firms, or diagnostic companies can provide access to cutting-edge technologies and clinical trial networks. Securing regulatory approvals can accelerate market entry. Targeting underserved populations and offering affordable generics or biosimilars can also create competitive advantages.
- Building strong distribution channels, engaging with neurologists and hospitals, and leveraging digital health platforms for patient outreach are essential for market penetration. Finally, investing in robust clinical trials and gathering real-world evidence helps establish credibility and support long-term growth in this highly specialized and evolving market.
North America Epilepsy Treatment Drugs Market Companies
Few of the prominent players operating in the North America epilepsy treatment drugs industry include:
- AbbVie
- Bausch Health Companies
- Dr. Reddy’s Laboratories
- Eisai
- GSK
- Jazz Pharmaceuticals
- Lupin Pharmaceuticals
- Neurelis
- Novartis
- Pfizer
- Sanofi
- SK Biopharmaceuticals
- Sumitomo Pharma
- Sun Pharmaceutical Industries
- UCB
- Novartis
Novartis strengthens its position in the epilepsy drug market through innovative therapies, strategic launches, and research and development focused on advanced antiepileptic drugs, aiming to improve seizure control and patient quality of life.
Pfizer drives innovation in epilepsy treatment through advanced second- and third-generation drugs, strategic launches, and sustained investment in neuroscience, strengthening its global leadership and improving patient outcomes.
Dr. Reddy’s Laboratories, through its German subsidiary Betapharm Arzneimittel GmbH, plays a vital role in providing high-quality generic medicines for epilepsy treatment.
North America Epilepsy Treatment Drugs Industry News:
- In April 2025, Neurelis received FDA approval for VALTOCO (diazepam nasal spray) for short-term treatment of seizure clusters in patients aged 2 years and older. Recognized as clinically superior to rectal diazepam gel and granted orphan drug exclusivity, this approval strengthens Neurelis’ position in emergency epilepsy care and enhances its credibility with healthcare providers.
The North America epilepsy treatment drugs market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Drug Class
- First-generation
- Second-generation
- Third-generation
Market, By Type
- Branded
- Generics
Market, By Route of Administration
- Oral
- Nasal
- Injectable
- Rectal
Market, By Age Group
- Pediatric
- Adult
Market, By Seizure Type
- Focal seizure
- Generalized seizure
- Combined seizure
Market, By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
The above information is provided for the following countries:
- U.S.
- Canada
Frequently Asked Question(FAQ) :
Who are the key players in the North America epilepsy treatment drugs market?
Key players include AbbVie, Bausch Health Companies, Dr. Reddy’s Laboratories, Eisai, GSK, Jazz Pharmaceuticals, Lupin Pharmaceuticals, Neurelis, and Novartis.
What are the upcoming trends in the North America epilepsy treatment drugs industry?
Key trends include increased funding for epilepsy programs, the establishment of specialized networks like the Pediatric-Onset Epilepsies Network, and advancements in antiepileptic drug development.
Which country dominates the North America epilepsy treatment drugs market?
The United States dominated the market with a 90.1% share in 2024. The U.S. market was valued at USD 3.1 billion in 2024.
What was the valuation of the oral route of administration segment?
The oral route of administration segment generated USD 2.15 billion in 2024, dominating the market with a CAGR of 4.7% expected over the forecast period.
Which age group leads the North America epilepsy treatment drugs market?
The adult segment led the market with a revenue of USD 2.48 billion in 2024 and is projected to grow at a CAGR of 4.3% during the forecast period.
How much revenue did the second-generation drug class segment generate?
The second-generation drug class segment generated the largest revenue share of 47.2% in 2024 and is projected to grow at a CAGR of 4.4% over the forecast period.
What is the market size of the North America epilepsy treatment drugs market in 2024?
The market size was USD 3.42 billion in 2024, with a CAGR of 4.4% expected through 2034, driven by supportive government policies, sustained funding, and advancements in drug development.
What is the projected value of the North America epilepsy treatment drugs market by 2034?
The market is expected to reach USD 5.25 billion by 2034, fueled by investments in healthcare infrastructure and the discovery of novel antiepileptic drugs.
What is the projected size of the North America epilepsy treatment drugs market in 2025?
The market is expected to reach USD 3.55 billion in 2025.
North America Epilepsy Treatment Drugs Market Scope
Related Reports


