Summary
Table of Content

Magnetic Nanoparticles for Hyperthermia Treatment Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Magnetic Nanoparticles for Hyperthermia Treatment Market Size
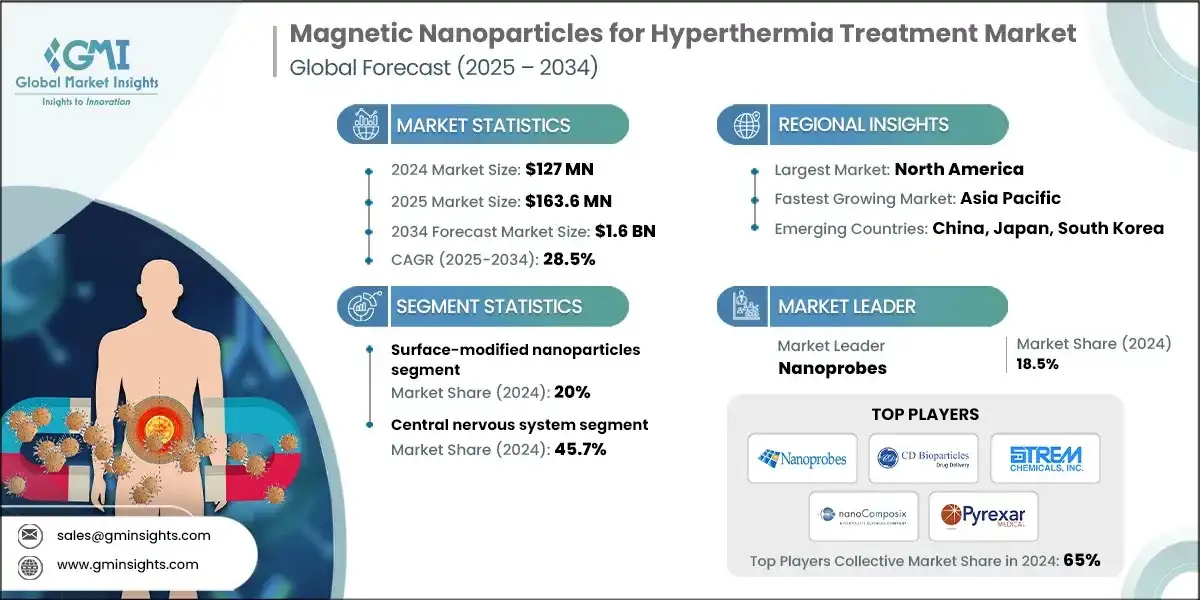
The global magnetic nanoparticles for hyperthermia treatment market was valued at USD 127 million in 2024 and reflects early but accelerating commercialization momentum. It is projected to expand from USD 163.6 million in 2025 to USD 1.6 billion by 2034, registering a 28.5% CAGR over 2025–2034, according to latest report published by Global Market Insights Inc.
To get key market trends
- Magnetic nanoparticles for hyperthermia treatment are a novel technique of cancer treatment using superparamagnetic iron oxide nanoparticles (SPIONs) and engineered nanoparticle systems that can produce localized heat when exposed to alternating magnetic fields. This localized hyperthermia selectively destroys cancer cells while protecting healthy tissue and is expected to produce better therapeutic outcomes than conventional treatments.
- Growing prevalence of treatment-resistant cancers and the urgent need for precision oncology solutions are driving unprecedented demand for magnetic hyperthermia technologies. The COVID-19 pandemic accelerated research investments in advanced cancer therapeutics, with central nervous system cancers and genitourinary cancers showing particularly strong adoption rates due to the technology's ability to cross biological barriers and target deep-seated tumors.
- Manufacturing complexities around nanoparticle synthesis, biocompatibility validation, and regulatory approval processes continue to challenge market expansion. However, significant advances in surface modification techniques, combination therapy protocols, and clinical trial methodologies are accelerating the path toward commercial viability and widespread clinical adoption.
Magnetic Nanoparticles for Hyperthermia Treatment Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 127 Million |
| Market Size in 2025 | USD 163.6 Million |
| Forecast Period 2025 - 2034 CAGR | 28.5% |
| Market Size in 2034 | USD 1.6 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Breakthrough advances in targeted cancer therapy | Magnetic nanoparticles enabling precise tumor targeting with minimal systemic toxicity |
| Rising demand for combination therapy protocols | Integration with radiation and chemotherapy improving treatment efficacy and patient outcomes |
| Technological advancement in nanoparticle engineering | Surface-modified and engineered systems enhancing biocompatibility and therapeutic effectiveness |
| Pitfalls & Challenges | Impact |
| Complex regulatory approval pathways and high development costs | Lengthy clinical trial requirements and specialized manufacturing limiting rapid market penetration |
| Opportunities: | Impact |
| Growing precision oncology market and personalized medicine trends | Increasing investment in targeted therapeutics driving adoption in premium cancer treatment centers. |
| Market Leaders (2024) | |
| Market Leaders |
18.5% market share |
| Top Players |
Collective market share is 65% in 2024 |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Country | China, Japan, South Korea |
| Future Outlook |
|
What are the growth opportunities in this market?
Magnetic Nanoparticles for Hyperthermia Treatment Market Trends
- Precision targeting and temperature control: Precision targeting and temperature control are changing the clinical value offer. Magnetic nanoparticles can heat tumors locally between 42 and 45 °C, which causes damage to cancer cells and sensitizes them to radiation and chemotherapy while sparing normal tissues. Controllability is the real driver here, feedback systems and MR-compatible formulations allow clinicians to see distribution and modulate heating in real time. That is in sharp contrast to the older hyperthermia methods which have less spatial selectivity, with targeted nanoparticles, the benefits-to-risk ratio becomes more attractive at sensitive sites. Clinical observations manifest remarkable SAR values and reproducible heating over realistic field ranges, allowing for probable response rates and repeatability.
- Surface engineering innovations- Surface engineering is transitioning from optional to mandatory. PEG stealthing for prolonged circulation, ligand/antibody conjugation for active targeting, and pH/enzyme-sensitive coatings for controlled release are now standard features in advanced systems. improved accumulation in the tumor and reduced RES clearance allow for wider therapeutic windows. And there's more-core size uniformity (about 10-15 nm for SPION cores) and hydrodynamic size optimization (often ~50-70 nm) correlate with better magnetic response and biodistribution. Medium- to long-term growth, with engineered nanoparticle systems showing the steepest adoption curves in the magnetic nanoparticles for hyperthermia treatment market.
- Closed-loop systems and real-time monitoring- Development pipelines have embraced closed-loop control into popular practice. Such imaging capabilities embedded in therapeutic payloads allow teams to visualize localization of the nanoparticles with simultaneous temperature rise and subsequently adjust AMF parameters to maintain thermal windows in the tissue. More predictable outcomes and fewer adverse events, which can support payer conversations and protocol standardization. Within ~0.5°C, feedback accuracy has been reported in the controlled models and is increasingly becoming the benchmark for design of systems.
Magnetic Nanoparticles for Hyperthermia Treatment Market Analysis
Learn more about the key segments shaping this market
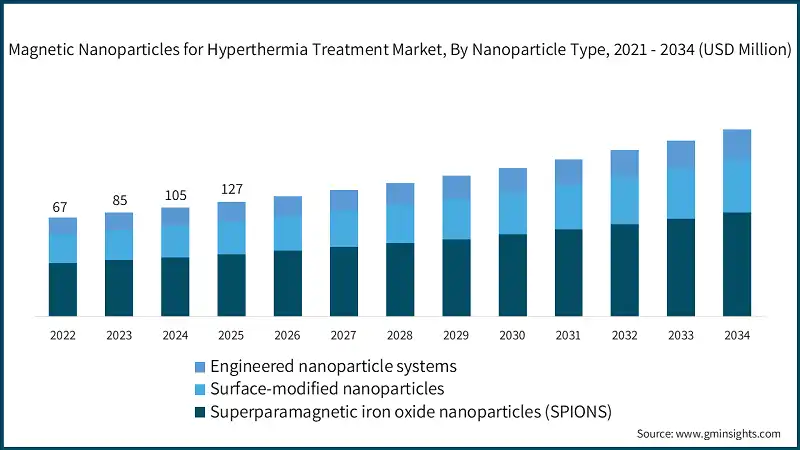
SPIONs lead the magnetic nanoparticles for hyperthermia treatment industry with an estimated 70.1% market share in 2024, underpinned by their safety profile, consistent magnetic response, and broad clinical familiarity. Growth at roughly 26.8% CAGR reflects ongoing improvements in core uniformity and magnetization, which stabilize SAR and enhance predictability. The industry also values SPION compatibility with existing imaging modalities and protocols, making them the default platform for many centers. Clinically, SPION core sizes near 10–15 nm and optimized hydrodynamic diameters have been associated with favorable biodistribution and heating performance. Because of this, SPIONs anchor the early scaling phase of the market.
Surface-modified nanoparticles represent about 20% market share today and are expanding at an estimated 32.1% CAGR as coatings move from passive stealth layers to active, disease-targeting interfaces. Features like PEG stealthing, antibody/ligand targeting, and stimuli-responsive shells boost tumor accumulation and selectivity, often improving the therapeutic index in combination regimens. Then there’s the engineered nanoparticle systems segment roughly 9.9% market share but growing near 35.4% CAGR packing multi-functionality such as drug delivery, imaging, and magnetic heating in a single platform. The premium they command is tied to superior performance and clinical workflow advantages, which can justify higher per-procedure costs where outcomes are meaningfully better.
Learn more about the key segments shaping this market
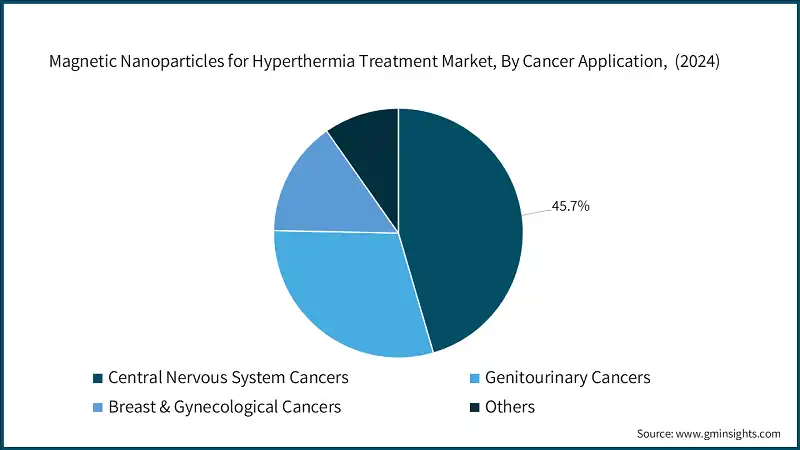
Central nervous system segment held 45.7% market share of the magnetic nanoparticles for hyperthermia treatment market, all due to the need for a minimally invasive approach in brain tumors. MR-visible formulations and catheter-based delivery provide very accurate dosing in glioblastoma and recurrent disease settings. The ANCHIALE glioblastoma study is an example of this drive, in which recruitment is ongoing to build outcome datasets for tumor control and safety endpoints. Clinical need keeps CNS-CNS applications at the top of the application mix. Hence, investments continue on protocols that emphasize homogeneous heating and neuroprotection planning.
Genitourinary cancers comprise about 30% market share and are foreseeably growing at about 31.8% CAGR, favorably assisted by anatomy for magnetic field application and thermometry. Hyperthermia is postulated to sensitize prostate tumors to radiation while ameliorating toxicities, thus improving localized control in select cohorts. Breast and gynecology cancers comprise around 15% market share, with the fastest growth, as superficial or accessible sites allow for consistent heating and more straightforward targeting strategies. The other avenue expected for increasing exploration involves an adjuvant capacity, whereby shrinking tumors pre-surgery will tantalize less invasive options.
Looking for region specific data?
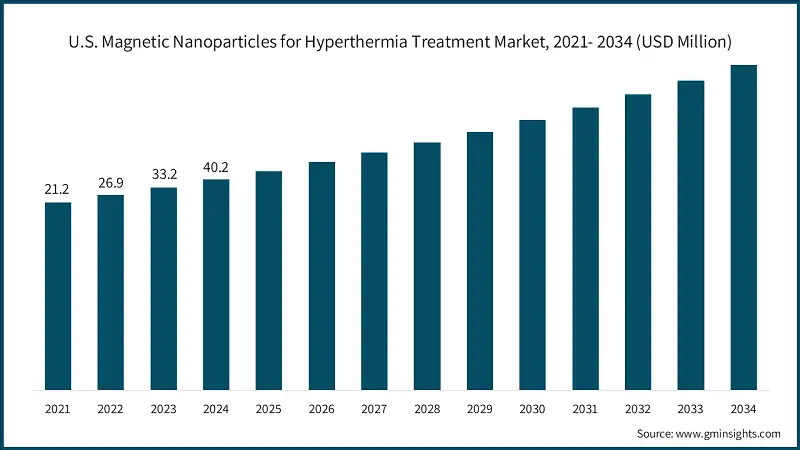
- The U.S. magnetic nanoparticles for hyperthermia treatment market reached value of USD 40.2 million in 2024, and is expected to grow up to almost USD 611.6 million by 2034. The U.S. market benefits from established hyperthermia systems like BSD-2000 (HDE H090002) and BSD-500 (PMA P820088), which provide infrastructure for nanoparticle-based protocols. Canada’s academic oncology hubs continue to expand hyperthermia programs alongside imaging-guided interventions. Because of this, device–nanoparticle integration and protocol standardization are advancing quickly, supported by active post-approval surveillance and training pathways.
- Europe holds roughly 35% market share of the magnetic nanoparticles for hyperthermia treatment market as clinical usage scales with supportive health-policy frameworks in 2024. Germany’s oncology centers have been early adopters of magnetic hyperthermia, with MR-guided planning and catheter-based delivery informing best practices reviewed across the literature. The UK, France, Italy, and Spain are expanding precision oncology infrastructure, which dovetails with theranostic nanoparticle platforms. Look at how trial ecosystems and procurement structures interact—coordinated adoption and standardized pathways often shorten time from positive data to clinical availability.
The magnetic nanoparticles for hyperthermia treatment market in China is projected to see substantial growth from 2025 to 2034.
- Asia Pacific is the fastest-growing region for the magnetic nanoparticles for hyperthermia treatment industry, propelled by oncology capacity build-outs and national precision-medicine initiatives in 2024. The China market is scaling research funding and translational programs, while the India market is adding advanced oncology centers that can host theranostic protocols. Japan’s focus on materials science and device miniaturization supports next-gen SPIONs and field generators reviewed in recent studies. The timing here favors rapid clinical uptake once flagship centers report durable outcome advantages.
The magnetic nanoparticles for hyperthermia treatment market in Brazil is expected to grow at a significant pace from 2025 to 2034.
- The Brazilian market demonstrates exceptional growth potential from 2025 to 2034, driven by the country's expanding oncology infrastructure and increasing cancer incidence rates. Brazil's robust healthcare system, anchored by leading cancer treatment centers like Instituto Nacional de Câncer (INCA) and Hospital Sírio-Libanês, creates favorable conditions for adopting advanced nanomedicine technologies and precision cancer therapies highly valued in global oncology markets. The emerging biotechnology sector and medical research institutions in Brazil are actively supporting the development of innovative hyperthermia treatment protocols and nanoparticle synthesis capabilities.
The magnetic nanoparticles for hyperthermia treatment market in Saudi Arabia is expected to grow at a significant pace from 2025 to 2034.
- Substantial growth will continue through 2025-2034 for the Saudi market, supported by Vision 2030's emphasis on healthcare sector transformation and medical innovation. The Kingdom's ambitious healthcare infrastructure investments, including the development of specialized cancer centers and precision medicine facilities, drive demand for cutting-edge nanomedicine technologies, creating opportunities for profitable adoption of magnetic hyperthermia systems in oncology applications.
- Saudi Arabia's strategic geographical position facilitates access to magnetic nanoparticle technologies from European and American manufacturers, while growing domestic demand for advanced cancer treatments provides substantial market opportunities. Specific Saudi investments in healthcare development and medical education, particularly within specialized oncology centers and research hospitals, ensure consistent demand for magnetic hyperthermia applications. The country's focus on becoming a regional medical tourism hub for cancer treatment further accelerates the adoption of innovative therapeutic technologies like magnetic nanoparticle hyperthermia systems.
Magnetic Nanoparticles for Hyperthermia Treatment Market Share
The top five companies in the market are CD Bioparticles, Nanoprobes Inc., Strem Chemicals, BSD Medical Corporation, and Pyrexar Medical which hold a combined 63% market share. In practical terms, specialized synthesis know-how, GMP scale-up, and device–nanoparticle integration create barriers that favor experienced suppliers. The magnetic nanoparticles for hyperthermia treatment market share of the leader, CD Bioparticles, stands near 15%, reflecting both breadth of formulations and tight collaboration with research hospitals. As protocols standardize, purchasers prefer vendors who can deliver clinical-grade consistency and provide technical support for AMF parameterization and thermometry workflows.
Competitive strategies tilt toward three priorities. First, platform breadth—vendors are building families of SPIONs and coated/functionalized variants to match tumor biology and dosing routes. Second, theranostics—incorporating imaging agents and feedback capabilities to reduce uncertainty and improve outcomes. Third, combination-therapy enablement—positioning nanoparticles to amplify chemo/radiation (and emerging immuno-oncology regimens) is becoming a central pitch. Literature support for SAR performance and safety parameterization helps vendors substantiate these claims in formulary and IRB discussions.
M&A and partnerships are also active. Larger medtech and biopharma organizations are scouting acquisitions and co-development deals to accelerate entry, while device makers with hyperthermia platforms collaborate on nanoparticle optimization. Notably, regulated hyperthermia systems with established post-approval surveillance give clinical teams a procedural backbone for nanoparticle integration. Expect IP to shape competition as patents on core synthesis and surface-engineering methods evolve. Vendors with defensible IP and proven GMP lines should consolidate position as the magnetic nanoparticles for hyperthermia treatment industry expands.
Magnetic Nanoparticles for Hyperthermia Treatment Market Companies
Major players operating in the magnetic nanoparticles for hyperthermia treatment industry are:
- Nanoprobes
- CD Bioparticles
- Strem Chemicals
- BSD Medical Corporation
- Pyrexar Medical
- Nano Composix
- Spherotech
Nanoprobes, Inc works in deep studies in nanomaterials chemistry within a biomedical focus, magnetic nanoparticles designed specifically for hyperthermia and imaging workflows. Its years of investment in surface functionalization and bioconjugation gives company competitive advantage in targeted delivery and theranostic designs, rendering it a perfect selection in the context of the translational research program. In other words, Nanoprobes has its customized strategy to maintain its interests in synthesis and very close partnerships with the academic oncology teams, especially to prove the protocols and accelerate clinical translation.
CD Bioparticles boasts a full arsenal of magnetic nanoparticles for hyperthermia treatment, from SPIONs and gold-coated magnetic particles to biotin-functionalized variants. They are all diversified into research and clinical-grade development. Sustained investment and continued development of R&D (including insistence on uniformity) can reduce the variability of survey results (SAR) and heat profiles because they all have specifications for consistency in magnetization and performance across lots. Lastly, the nanoparticle portfolio is broad enough for several application niches, enabling protocol-specific selection and price tiers.
Strem Chemicals makes the availability of quality high-purity iron oxide and specialty nanomaterials available to contributors and research hospitals in the sciences of materials. The emphasis on purity, reproducibility, and documentation meets the GMP and QA requirements for clinical translation in the industry. It serves to broaden the outlook of the company, which is into serving early-stage and scale-up demand in the magnetic nanoparticles for hyperthermia treatment market.
Nanocomposix is the leading provider of quality nanomaterials, specializing in the synthesis and customizing of magnetic nanoparticles applied in different biomedical and industrial applications. Focused on innovation and precision, the company has a super wide product offering, including superparamagnetic iron oxide nanoparticles (SPIONs) that are used for targeted drug delivery, imaging, and hyperthermia treatments. Nanocomposix is fully committed to quality by providing well-characterized and reproducible nanomaterials that meet standard industry requirements. Their expertise in surface modification and functionalization enables specific tailoring solutions for particular research and commercial needs. Support is provided to clients through an extensive package technical support securing the successful integration of nanomaterials into advanced applications. Hence, Nanocomposix is a trusted partner in the quickly changing corridors of nanotechnology.
Pyrexar Medical ties together this system in an approach that takes-not only end-user-friendly hyperthermia delivery platforms-but also training supplies. Incorporating device features with frequency/control MR compatibility (i.e., for performance targets of nanoparticle versus device) enhances reproducible results and wider institutional adoption. Strategic alliances are also being formed with nanoparticle suppliers to situate these systems well among their most important applications in the theranostic and combination-therapy protocols in the magnetic nanoparticles for hyperthermia treatment market.
Magnetic Nanoparticles for Hyperthermia Treatment Industry News
- In August 2024, CD Bioparticles closed at USD 25 million Series B to accelerate theranostic nanoparticle development and manufacturing scale-up. The investment, led by prominent healthcare venture capital firms, will enable the company to establish a 50,000 square-foot GMP manufacturing facility in Boston and expand their proprietary surface modification technology platform.
- In Jun 2024, Pyrexar Medical received FDA Breakthrough Device designation for a next-gen magnetic hyperthermia system aimed at prostate cancer treatment. The innovative system combines real-time MRI guidance with precision magnetic field control, enabling targeted hyperthermia delivery to prostate tumors while minimizing damage to surrounding healthy tissue.
- The magnetic nanoparticles for hyperthermia treatment market research report includes in-depth coverage of the industry, with estimates & forecast in terms of revenue (USD Million) from 2021 to 2034, for the following segments:
Market, By Nanoparticle Type
- Superparamagnetic iron oxide nanoparticles (SPIONS)
- Magnetite (fe3o4) core formulations
- Maghemite (γ-fe2o3) based systems
- Core-shell iron oxide structures
- Surface-modified nanoparticles
- Engineered nanoparticle systems
- Size-optimized particles (10-100 nm) for tissue penetration
- Multi-functional nanoparticles with imaging capabilities
- Biodegradable formulations for enhanced safety
Market, By Cancer Application
- Central nervous system cancers
- Glioblastoma multiforme (GBM) treatment
- Recurrent brain tumor CE marking precedent
- Genitourinary cancers
- Prostate cancer localized hyperthermia
- Cervical carcinoma with radiation therapy combinations
- Breast & gynecological cancers
- Superficial breast cancer
- Ovarian cancer peritoneal
- Others
Market, By Treatment Modality
- Standalone magnetic hyperthermia
- Combination with radiation therapy
- Combination with chemotherapy
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- Middle East & Africa
- UAE
- Saudi Arabia
- South Africa
- Rest of Middle East & Africa
Frequently Asked Question(FAQ) :
Who are the key players in the magnetic nanoparticles for hyperthermia treatment market?
Key players include Nanoprobes, CD Bioparticles, Strem Chemicals, BSD Medical Corporation, Pyrexar Medical, Nano Composix, and Spherotech.
What are the upcoming trends in the magnetic nanoparticles for hyperthermia treatment industry?
Key trends include advancements in nanoparticle engineering, integration with combination therapies, and the development of MR-visible formulations for precise dosing.
What is the growth outlook for the U.S. magnetic nanoparticles for hyperthermia treatment market from 2025 to 2034?
The U.S. market is expected to grow from USD 40.2 million in 2024. Growth is driven by established hyperthermia systems and advancements in device–nanoparticle integration.
What was the market share of the central nervous system (CNS) segment in 2024?
The CNS segment accounted for 45.7% of the market in 2024, supported by the demand for minimally invasive approaches in brain tumor treatments.
What was the valuation of the SPION segment in 2024?
SPIONs held a 70.1% market share in 2024, driven by their safety profile, consistent magnetic response, and compatibility with existing imaging modalities.
What is the projected value of the magnetic nanoparticles for hyperthermia treatment market by 2034?
The market is expected to reach USD 1.6 billion by 2034, fueled by increasing investment in precision medicine and expanding clinical trial infrastructure.
What is the current magnetic nanoparticles for hyperthermia treatment market size in 2025?
The market size is projected to reach USD 163.6 million in 2025.
What is the market size of the magnetic nanoparticles for hyperthermia treatment industry in 2024?
The market size was USD 127 million in 2024, with a CAGR of 28.5% expected through 2034, driven by advancements in targeted cancer therapies and nanoparticle engineering.
Magnetic Nanoparticles for Hyperthermia Treatment Market Scope
Related Reports


