Summary
Table of Content

Influenza Diagnostic Tests Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Influenza Diagnostic Tests Market Size
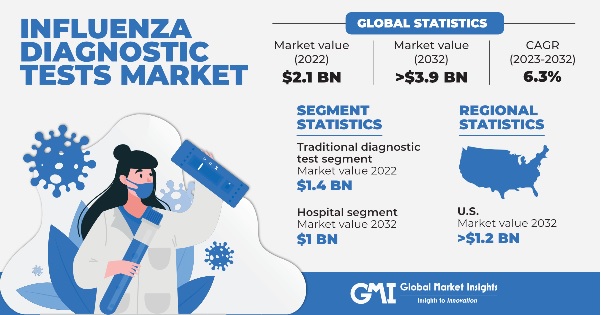
Influenza Diagnostic Tests Market size was valued at around USD 2.1 billion in 2022 and is estimated to witness 6.3% CAGR between 2023 and 2032. The growing prevalence of influenza, rising demand for rapid diagnostic tests, and increase in R&D activities are some of the key factors driving the market progression.
To get key market trends
According to data from the World Health Organization, in 2023, there were around a billion cases of seasonal influenza annually, including 3–5 million cases of severe illness. Moreover, influenza causes 290,000 to 650,000 respiratory deaths annually around the globe. The rise in the incidence of influenza cases, especially during seasonal outbreaks, has led to an increased demand for accurate and rapid influenza diagnostic tests (RIDT) for timely identification and management of the infection. As a result, the rising influenza cases coupled with the high mortality rate are expected to drive the market progress.
Influenza diagnostic tests refer to a range of medical procedures and technologies designed to identify the presence of influenza viruses in individuals suspected of having an influenza infection. These tests play a crucial role in the timely and accurate diagnosis of influenza, allowing healthcare professionals to initiate appropriate interventions, treatment, and public health measures.
Influenza Diagnostic Tests Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2022 |
| Market Size in 2022 | USD 2.1 Billion |
| Forecast Period 2023 to 2032 CAGR | 6.3% |
| Market Size in 2032 | USD 3.9 Billion |
| Key Market Trends | |
| Growth Drivers |
|
| Pitfalls & Challenges |
|
What are the growth opportunities in this market?
The market faces the potential risk of hindered growth due to stringent regulatory guidelines. These guidelines are implemented to uphold the safety and efficacy of diagnostic solutions, often resulting in lengthy approval processes for new influenza diagnostic tests. Companies may experience delays in bringing their products to market due to the comprehensive evaluations required by regulatory agencies. This, in turn, can impede the timely availability of innovative diagnostic solutions, potentially hindering the business landscape.
COVID-19 Impact
The COVID-19 pandemic significantly impacted the influenza diagnostic tests market in 2020. The coexistence of COVID-19 and influenza heightened the global demand for accurate testing. Governments emphasized surveillance for both infections, altering testing strategies. Supply chain disruptions affected test kit production and distribution, leading to operational challenges at testing facilities. Despite a modest decline in growth initially, the market swiftly rebounded due to increased awareness among patients and caregivers.
Influenza Diagnostic Tests Market Trends
Rising advancements in influenza diagnostic tests intended to improve the speed, accuracy, and efficiency in the detection of influenza viruses will spur the business growth.
- The adoption of molecular diagnostics technique, particularly polymerase chain reaction (PCR) technology, have significantly enhanced the sensitivity and specificity of influenza diagnostic tests. Real-time PCR technologies allow for the rapid and accurate detection of influenza virus genetic material, enabling early diagnosis and effective management.
- Moreover, technological progress has led to the development of multiplex assays that can simultaneously detect multiple respiratory pathogens, including different strains of influenza viruses. These assays enhance the efficiency of testing and provide comprehensive information, aiding in the differential diagnosis of respiratory infections.
- These advancements not only enhance the accuracy and efficiency of influenza diagnostic tests but also contribute to a more robust and adaptable diagnostic ecosystem. Thus, technological advancements in influenza diagnostic tests are expected to drive the market development.
Influenza Diagnostic Tests Market Analysis

Learn more about the key segments shaping this market
Based on test type, the market is segmented into traditional diagnostic test and molecular diagnostic assay. The traditional diagnostic test segment was valued at around USD 1.4 billion in 2022 and is expected to exhibit a robust growth trend throughout the analysis period.
- Traditional methods have been widely utilized for their ease of use, quick results, and cost-effectiveness. These tests include a rapid influenza diagnostic test (RIDT), direct fluorescent antibody test (DFAT), viral culture, and serological assay. These tests detect specific viral proteins (antigens) in respiratory specimens, providing quick results within minutes. The simplicity and rapid turnaround time of antigen tests make them well-suited for point-of-care settings, including clinics, urgent care centers, and primary care offices.
- The growth of traditional diagnostic tests is influenced by factors such as accessibility, cost-effectiveness, and the ability to deliver rapid results in various healthcare settings. While newer technologies, including molecular methods and point-of-care nucleic acid amplification tests, have gained prominence, traditional tests continue to play a crucial role, especially in resource-limited environments and during seasonal outbreaks where a large number of tests are required quickly.

Learn more about the key segments shaping this market
Based on end-use, the influenza diagnostic tests market is segmented into hospitals, diagnostics centers, research laboratories, and others. The hospital segment is projected to reach more than USD 1 billion by the end of 2032.
- The surging use of influenza diagnostic tests in hospital settings delivering affordable and efficient diagnosis is estimated to surge the patient preference for these healthcare facilities. Hospitals often have centralized and well-equipped laboratories that can handle a high volume of diagnostic tests. Centralized testing facilities within hospitals enable efficient processing of influenza diagnostic tests, especially during peak seasons or outbreaks.
- Moreover, hospitals play a pivotal role in surveillance and outbreak management. During influenza season or in the event of an outbreak, hospitals conduct widespread testing to monitor the prevalence of the virus, implement infection control measures, and guide public health interventions. Thus, accessibility to better diagnosis & treatment coupled with the rising prevalence of influenza and associated conditions is estimated to boost patient visits to hospitals, thereby propelling the segment revenue.

Looking for region specific data?
The U.S. dominated the North American influenza diagnostic tests market with a significant market share in 2022 and is anticipated to expand at a notable pace to reach more than USD 1.2 billion by 2032.
- This notable market share can be attributed to various factors, including robust healthcare infrastructure, R&D initiatives, and high disease burden and seasonal outbreaks, among other key drivers.
- The U.S. boasts an advanced and well-established healthcare infrastructure, characterized by state-of-the-art medical facilities, laboratories, and diagnostic centers. The country's sophisticated healthcare system facilitates the widespread availability and adoption of influenza diagnostic tests.
- Moreover, the U.S. is a hub for medical research and development, with ongoing initiatives aimed at improving diagnostic capabilities. Research institutions, universities, and private companies in the country are at the forefront of innovations in influenza diagnostics, leading to the introduction of cutting-edge technologies.
Influenza Diagnostic Tests Market Share
The influenza diagnostic tests industry is fragmented in nature, with companies competing to offer superior influenza diagnostic tests in this business space. Prominent players such as F. Hoffmann-La Roche Ltd, Abbott, and Becton Dickinson and Company Inc hold a significant share in this market. These companies are diligently directing their efforts towards continuous type innovation to gain substantial market share.
Some of the eminent market participants operating in the influenza diagnostic tests industry include:
- F. Hoffmann-La Roche Ltd
- Abbott
- Becton
- Dickinson
- Company
- Coris BioConcept
- DiaSorin SpA
- Meridian Bioscience Inc
- Quidel Corporation
- Sekisui Diagnostics
- Thermo Fischer Scientific Inc
- Hologic Inc
Influenza Diagnostic Tests Industry News:
- In June 2022, BD (Becton, Dickinson, and Company) announced that the BD MAX Respiratory Viral Panel (RVP), a new molecular diagnostic combination test for SARS-CoV-2, Influenza A + B and Respiratory Syncytial Virus (RSV), has been CE marked. The test uses a single nasal swab or a single nasopharyngeal swab sample to determine if a patient has COVID-19 or the flu or RSV. This product launch may help the company to enhance their market presence and generate increased business revenue.
- In February 2019, Abbott announced the launch of its rapid influenza diagnostic test (RIDT), BinaxNOW Influenza A & B Card 2. The reformulated test has been granted approval by the U.S. Food and Drug Administration (FDA) for use with Abbott's DIGIVAL diagnostic reader for the rapid detection of influenza virus. This product helped the company to expand its product portfolio and acquire enhanced customer base.
Influenza diagnostic tests market research report includes an in-depth coverage of the industry with estimates & forecasts in terms of revenue in USD (million) from 2018 to 2032 for the following segments:
By Test Type, 2018 - 2032 (USD Million)
- Traditional diagnostic test
- Rapid influenza diagnostic test (RIDT)
- Direct fluorescent antibody test (DFAT)
- Viral culture
- Serological assay
- Molecular diagnostic assay
- RT-PCR
- Loop-mediated isothermal amplification-based Assay (LAMP)
- Nucleic acid sequence-based amplification test (NASBAT)
- Simple amplification-based assay (SAMBA)
- Other molecular diagnostic assays
By End-use, 2018 - 2032 (USD Million)
- Hospitals
- Diagnostics centers
- Research laboratories
- Other end-users
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Frequently Asked Question(FAQ) :
How big is the U.S. influenza diagnostic tests industry?
The U.S. influenza diagnostic tests market is anticipated to expand at a notable pace to reach more than USD 1.2 billion by 2032, attributed to robust healthcare infrastructure, R&D initiatives, and high disease burden, and seasonal outbreaks.
Which companies define the competitive landscape of influenza diagnostic tests market?
F. Hoffmann-La Roche Ltd, Abbott, Becton, Dickinson, and Company, Coris BioConcept, DiaSorin SpA, Meridian Bioscience Inc., Quidel Corporation, Sekisui Diagnostics, Thermo Fischer Scientific Inc., and Hologic Inc.
What is the size of the influenza diagnostic tests market?
The influenza diagnostic tests market was valued at around USD 2.1 billion in 2022 and is estimated to reach over USD 3.9 billion by 2032, backed by the growing prevalence of influenza, rising demand for rapid diagnostic tests, and increase in R&D activities.
Why are traditional influenza diagnostic tests gaining traction?
The traditional diagnostic test segment was valued at around USD 1.4 billion in 2022 and is expected to exhibit a robust growth trend through 2032, owing to their ease of use, quick results, and cost-effectiveness.
Influenza Diagnostic Tests Market Scope
Related Reports


