Summary
Table of Content

Inflammatory Bowel Disease Treatment Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Inflammatory Bowel Disease Treatment Market Size
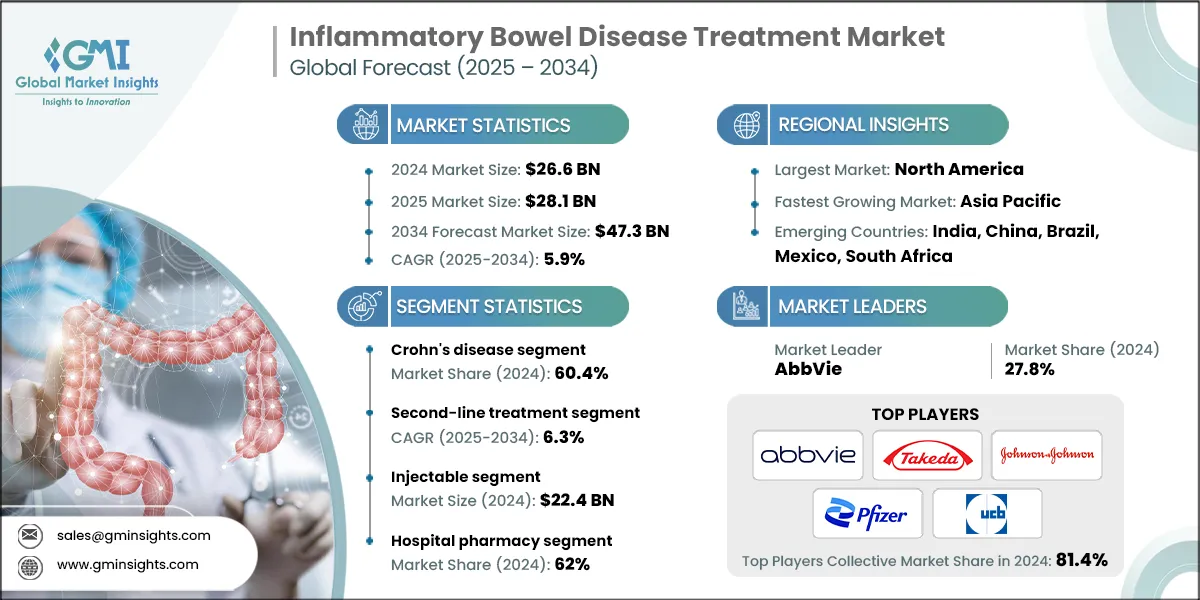
The global inflammatory bowel disease treatment market was valued at USD 26.6 billion in 2024. The market is expected to grow from USD 28.1 billion in 2025 to USD 47.3 billion in 2034, at a CAGR of 5.9% during the forecast period, according to the latest report published by Global Market Insights Inc. The high market growth is attributed to several factors, including increasing prevalence of IBD, favorable reimbursement policies, and growing awareness and early diagnosis of IBD symptoms.
To get key market trends
IBD treatment focuses on managing chronic inflammation of the gastrointestinal tract, primarily in conditions such as Crohn’s disease and ulcerative colitis. Therapeutic approaches include a range of medications such as aminosalicylates, corticosteroids, IL inhibitors, and biologics, which aim to reduce inflammation, control symptoms, and maintain remission.
Leading players in the IBD treatment market include Takeda, AbbVie Inc., and Johnson & Johnson. These companies maintain their competitive edge through continuous product innovation, global market presence, and significant investments in research and development.
Inflammatory Bowel Disease Treatment Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 26.6 Billion |
| Forecast Period 2025 - 2034 CAGR | 5.9% |
| Market Size in 2034 | USD 47.3 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of IBD | Accelerates the need for innovative therapies and expands the patient base for treatment providers. |
| Technological advancements | Drive precision medicine and enable the development of minimally invasive diagnostic and therapeutic tools. |
| Favorable reimbursement policies | Encourage broader adoption of advanced treatments by reducing financial barriers for patients. |
| Growing awareness and early diagnosis of IBD symptoms | Promotes proactive disease management and improves long-term patient outcomes. |
| Pitfalls & Challenges | Impact |
| Stringent regulatory scenario | Delays market entry for novel therapies and increases compliance costs for manufacturers. |
| High cost of treatment | Restricts access for low-income populations and strains healthcare budgets. |
| Opportunities: | Impact |
| Rising adoption of biologics and targeted therapies | Spurs growth in personalized medicine and enhances treatment efficacy. |
| Expansion in emerging markets | Unlocks new growth avenues through increased healthcare investment and rising disease awareness. |
| Market Leaders (2024) | |
| Market Leaders |
27.8% Market Share |
| Top Players |
Collective market share in 2024 is 81.4% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | India, China, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
The market has increased from USD 23.1 billion in 2021 and reached USD 25.5 billion in 2023. The market growth was driven by the increasing global prevalence of inflammatory bowel disease (IBD), particularly Crohn’s disease and ulcerative colitis, fueled by lifestyle changes, dietary habits, and environmental factors. As a result, the demand for advanced and targeted pharmacological therapies for IBD has significantly increased.
The high market growth is attributed to the increasing prevalence of inflammatory bowel disease (IBD), advancements in biologic therapies, rising awareness and diagnosis rates, growing demand for personalized medicine, and expanding healthcare infrastructure in emerging markets, among other contributing factors.
The global incidence and prevalence of inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, continue to rise. For instance, according to the Centers for Disease Control and Prevention (CDC), in 2023, approximately 3 million adults in the U.S. were diagnosed with IBD, up from 2.4 million in 2021.
The WHO reported that urbanization rates reached 56.6% globally in 2023, correlating with increased IBD cases, particularly in developing regions. A 2023 National Institutes of Health study revealed that sedentary behaviour among adults increased by 28% between 2021 and 2023, while processed food consumption rose by 12% during the same period. The growing number of diagnosed patients drives the demand for treatment options, expanding the market.
In addition, pharmaceutical companies have been ramping up their investments in research and development, leading to innovation across the IBD treatment landscape. Strategic collaborations, mergers, and acquisitions are helping accelerate the development of new therapies. Similarly, growing awareness among both patients and healthcare professionals is encouraging earlier diagnosis and better disease management. Support from governments, including funding programs, streamlined regulatory pathways, and favorable reimbursement policies, is making it easier for patients to access cutting-edge treatments.
Inflammatory bowel disease (IBD) treatment is the medical management of chronic gastrointestinal tract inflammatory conditions, including mainly Crohn's disease and ulcerative colitis. Treatment is to minimize inflammation, control symptoms, and induce long-term remission with drugs like aminosalicylates, corticosteroids, immunomodulators, biologics, and targeted small molecules in conjunction with diet, lifestyle, and occasionally surgery.
Inflammatory Bowel Disease Treatment Market Trends
Technological advancements are transforming the landscape of IBD treatment by enabling more precise, effective, and patient-friendly therapies. Innovations in drug development, diagnostics, digital health, and disease monitoring are collectively driving improved clinical outcomes and market growth.
- Advances in immunology and molecular biology have facilitated the development of biologics that target specific inflammatory pathways, including IL-12/23, IL-23, integrins, and TNF-α. According to the U.S. Food and Drug Administration (FDA), the approval of biologics increased by 35% between 2021 and 2023, reflecting the growing emphasis on targeted therapies.
- These treatments offer greater effectiveness and fewer side effects compared to conventional systemic therapies. The targeted nature of biologics enables physicians to customize treatment based on disease severity and patient response, leading to improved disease management.
- In addition, innovative drug delivery technologies such as delayed-release capsules, sustained-release injectables, and subcutaneous autoinjectors are enhancing the bioavailability and safety of IBD treatments. These technologies help reduce dosing frequency and adverse effects while enabling more consistent therapeutic exposure, making them particularly valuable for chronic treatment regimens.
- Thus, these technological innovations are playing a pivotal role in advancing IBD care by making treatments more effective, personalized, and accessible. As these technologies continue to evolve, they are poised to redefine disease management strategies and unlock new opportunities for stakeholders across the IBD treatment market.
Inflammatory Bowel Disease Treatment Market Analysis
Learn more about the key segments shaping this market
The global market was valued at USD 23.1 billion in 2021. The market size reached USD 25.5 billion in 2023, from USD 24.5 billion in 2022.
Based on the treatment type, the inflammatory bowel disease treatment market is segmented into Crohn's disease and ulcerative colitis. The Crohn's disease segment has asserted its dominance in the market, securing a significant market share of 60.4% in 2024, driven by the rising global prevalence, improved diagnostic capabilities, and the growing adoption of advanced biologics and targeted therapies that enhance long-term disease management and patient outcomes. The segment is expected to exceed USD 30.8 billion by 2034, growing at a CAGR of 6.7% during the forecast period. On the other hand, the ulcerative colitis bags segment is expected to grow with a CAGR of 4.6%. The growth of this segment can be attributed to the rising global prevalence, increasing awareness of disease symptoms, and expanding access to advanced treatment options, particularly in emerging regions.
- The rising global prevalence of Crohn's disease, particularly in developed regions, is a key factor driving the growth of this segment. Recent studies highlight a noticeable increase in cases among younger individuals, with most diagnoses occurring during adolescence or early adulthood, necessitating long-term treatment and continuous medical follow-up.
- Crohn's disease is typically more severe and complex than ulcerative colitis, as it can affect any part of the gastrointestinal tract and penetrate deeper layers of the bowel wall. This complexity often requires advanced treatment approaches, including immunosuppressive drugs and biologics, which contribute to its higher market value.
- Recent advancements in biologic therapies and small molecule drugs, such as JAK inhibitors and anti-integrin agents, have significantly transformed disease management. The growing use of combination therapies, therapeutic drug monitoring, and personalized treatment plans has further improved clinical outcomes and fueled segment growth.
- Additionally, clinicians are increasingly tailoring therapies based on individual biomarkers, genetic profiles, and disease behavior, enabling a more precise match between treatment and disease subtypes. Precision-guided therapy reduces trial-and-error prescribing, limits exposure to ineffective drugs, and enhances long-term disease control with fewer complications.
Based on drug class, the inflammatory bowel disease treatment market is segmented as first-line treatment, second-line treatment, and combination therapy. The second-line treatment segment dominated the market in 2024, accounting for USD 19.8 billion, and is anticipated to grow at a CAGR of 6.3% during the forecast period.
- Second-line treatment choices are becoming increasingly personalized, with sequencing guided by prior biologic exposure, side effects, and biomarker profiles. Clinicians are increasingly using therapeutic drug monitoring and disease phenotype to select the most appropriate follow-up therapy. This individualized approach helps improve outcomes and reduce unnecessary treatment cycling.
- In second-line therapy, physicians often combine biologics with immunomodulators to enhance treatment efficacy and minimize immune reactions against the drugs. According to the U.S. Food and Drug Administration (FDA), approximately 65% of inflammatory bowel disease patients received combination therapy with biologics and immunomodulators in 2023.
- Treatment typically follows a step-up approach, where medication intensity is increased based on disease severity and patient response. This strategy aims to control symptoms effectively while reducing the risk of long-term complications.
- The first-line treatment segment is expected to see significant growth over the forecast period. Traditionally dominated by amino salicylates, corticosteroids, and immunosuppressants, this segment is evolving with the integration of newer, more targeted therapies aimed at improving efficacy and reducing side effects from the outset. Growing emphasis on early diagnosis and treat-to-target approaches is encouraging the adoption of more personalized, front-loaded treatment regimens.
- The combination therapy segment holds the second position in the drug class segment and is anticipated to grow at a CAGR of 5.6% over the forecast period.
- Combination therapy in IBD refers to the use of two or more medications at the same time, often pairing a biologic with an immunomodulator, such as anti-TNF drugs combined with thiopurines. The aim of this treatment method is to improve how well the treatment works, improve the drug's durability, and lower the chances of the body developing resistance to biologics, especially anti-TNF drugs like infliximab and adalimumab.
Based on route of administration, the inflammatory bowel disease treatment market is segmented into injectable, oral, and rectal. The injectable segment dominated the market in 2024, accounting for USD 22.4 billion, and is anticipated to grow at a CAGR of 5.8% during the forecast period.
- Injectable biologics remain the cornerstone of IBD treatment, particularly in moderate to severe cases. Drugs targeting TNF-α, IL-12/23, and integrins are widely used to induce and maintain remission. Their proven efficacy and long-standing clinical use have made them first-line options in many treatment guidelines, especially for Crohn’s disease and ulcerative colitis.
- There is a growing preference for subcutaneous (SC) injections over intravenous (IV) infusions due to increased patient convenience and reduced hospital dependency. SC formulations enable at-home self-administration, improving treatment adherence and helping to lower healthcare costs. Many newer biologics are being developed or reformulated to support SC delivery.
- Additionally, the availability of injectable biosimilars has expanded significantly, offering cost-effective alternatives to originator biologics. These biosimilars are driving competition, increasing patient access, and supporting earlier treatment initiation. Payers are increasingly favoring biosimilars in formularies, accelerating their adoption across hospital and outpatient settings.
- The oral segment holds the second position in the application segment and is anticipated to grow at a CAGR of 6.7% over the forecast period. The growth of this segment is attributed to the increasing patient preference for non-invasive and convenient drug administration, rising adoption of oral small molecule therapies, and advancements in formulation technologies that enhance bioavailability and treatment adherence.
- Rectal segment was valued at USD 611.9 million in 2024 and is expected to grow at a CAGR of 5.2%. The growth of this segment is driven by its effectiveness in delivering localized treatment for distal ulcerative colitis, increasing adoption of patient-friendly formulations, and rising demand for targeted therapies that minimize systemic side effects.
Learn more about the key segments shaping this market
Based on distribution channel, the inflammatory bowel disease treatment market is classified into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market with a revenue share of 62% in 2024, owing to the availability of multidisciplinary care, access to advanced diagnostics, and the management of severe IBD cases that often require hospitalization, surgical intervention, and immediate access to biologics and specialty drugs.
- Hospital pharmacies play a central role in the evolving inflammatory bowel disease (IBD) treatment landscape, supported by their ability to handle complex therapies, manage high-cost biologics, and coordinate multidisciplinary care. These settings have become critical hubs for delivering advanced treatments, particularly those requiring in-office administration, close monitoring, or infusion services.
- Modern hospital pharmacies increasingly operate within integrated care frameworks, working alongside gastroenterologists, dietitians, and nursing teams to provide comprehensive, patient-centric management for Crohn’s disease and ulcerative colitis. This coordination improves adherence, enhances treatment outcomes, and ensures safer handling of immunosuppressants and biologics.
- The retail pharmacy segment has captured the second position in the distribution channel segment and is expected to grow at a CAGR of 6.1% during the forecast period. This growth is driven by the increasing availability of specialty IBD medications, enhanced patient convenience, and expanding access to chronic disease management solutions through retail pharmacies.
- The online pharmacy segment was valued at USD 2.3 billion in 2024 and is expected to witness steady growth in the forecast period. This is driven by the increasing adoption of digital health platforms, rising consumer preference for home delivery of medications, and the convenience of accessing specialty IBD treatments through secure and regulated e-commerce channels.
Looking for region specific data?
North America dominated the global inflammatory bowel disease treatment market with the highest market share of 65.7% in 2024.
- The U.S. and Canada report some of the highest incidence and prevalence rates of Crohn’s disease and ulcerative colitis globally, creating strong demand for effective treatment options. Widespread availability and early access to advanced biologics, biosimilars, and small-molecule drugs have significantly boosted treatment adoption across the region.
- Robust reimbursement frameworks and insurance coverage further encourage patients to opt for costly but highly effective therapies, driving revenue growth. In addition, the presence of leading pharmaceutical and biotechnology companies actively engaged in developing novel IBD drugs fosters continuous innovation and market expansion.
- Clinical research activity in North America is strong, with multiple ongoing trials aimed at improving treatment efficacy and safety profiles. Growing awareness among patients and healthcare providers about early diagnosis and management of IBD also contributes to rising treatment rates.
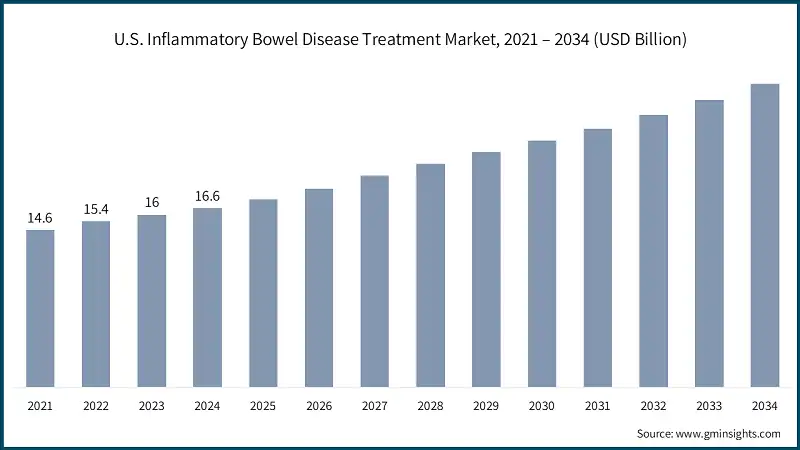
The U.S. inflammatory bowel disease treatment market was valued at USD 14.6 billion and USD 15.4 billion in 2021 and 2022, respectively. The market size reached USD 16.6 billion in 2024, growing from USD 16 billion in 2023, and is anticipated to grow at a CAGR of 5.5% between 2025 to 2034.
- The growing number of IBD cases, particularly Crohn’s disease and ulcerative colitis, across the U.S. continues to be a major factor driving market expansion. This rising disease burden is being amplified by greater public awareness, earlier detection, and improved access to specialized care centers nationwide. The widespread use of biologics and targeted therapies has transformed how IBD is managed, offering better symptom control and longer periods of remission.
- Meanwhile, innovations in drug delivery, like subcutaneous injections and extended-release formulations, are helping improve patient adherence and overall treatment outcomes. The market is also benefiting from a stronger emphasis on personalized medicine, where clinicians are increasingly using biomarkers and genetic profiling to tailor therapies to individual patient needs.
Europe inflammatory bowel disease treatment market accounted for USD 5.3 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- The most significant of these is the high and increasing incidence of IBD in the region, especially in Western Europe, where nations such as the UK, Germany, and France have some of the highest incidence rates in the world. A surging population and growing consciousness of gastrointestinal wellness are driving diagnosis at an earlier stage and long-term management of the disease.
- Further, Europe's mature healthcare system and conducive reimbursement environment enable patient access to more intensive therapies like biologics and biosimilars, which are extensively used as effective treatments for moderate to severe IBD. Innovating investments in research and development by pharma companies as well as public health organizations are speeding up the approval of innovative, targeted therapies with enhanced efficacy and safety.
Germany dominates the European inflammatory bowel disease treatment market, showcasing strong growth potential.
- Germany’s robust pharmaceutical industry, with global players and biotech firms actively engaged in developing next-generation biologics and biosimilars, further fuels market growth.
- Favorable reimbursement policies and high healthcare spending also make advanced therapies more accessible, encouraging patients and providers to adopt cutting-edge treatments. Moreover, the increasing focus on personalized medicine and precision therapies is driving the integration of targeted treatment options tailored to patient-specific needs.
- Rising clinical trial activity and collaborations between academic institutions, pharma companies, and contract research organizations (CROs) further enhance innovation in the market.
The Asia Pacific inflammatory bowel disease treatment market is anticipated to grow at the highest CAGR of 7.6% during the analysis timeframe.
- The Asia-Pacific region, particularly China, India, South Korea, and Japan, is witnessing a rising incidence of IBD, driven by urbanization, dietary changes (including high-fat and low-fiber intake), and environmental pollution.
- According to the National Health Commission of China, IBD cases rose by 8.4% in 2023 compared to the previous year. These lifestyle and environmental changes affect gut microbiota and immune responses, contributing to a growing patient pool and escalating demand for IBD therapies.
- Governments across the region are expanding tertiary care centers and gastroenterology services, improving access to diagnostics and treatment.
- The increasing availability of specialists in urban and semi-urban areas has led to more timely diagnoses and better therapeutic outcomes, thus driving pharmaceutical market expansion in previously underserved segments.
China inflammatory bowel disease treatment market is estimated to grow with a significant CAGR, in the Asia Pacific market.
- China is poised to witness strong growth in the inflammatory bowel disease (IBD) treatment market, driven by rising disease prevalence, increasing healthcare investments, and rapid adoption of advanced therapies. The country has seen a steady increase in cases of Crohn’s disease and ulcerative colitis, attributed to changing dietary habits, urbanization, and lifestyle factors, which has created a substantial demand for effective treatment options.
- Growing awareness among patients and healthcare providers about the importance of early diagnosis and management of IBD is also accelerating the uptake of innovative therapies. China’s government is actively investing in healthcare infrastructure and expanding reimbursement coverage for biologics and targeted therapies, making advanced treatment options more accessible.
- The presence of leading domestic and international pharmaceutical companies is fostering clinical trials and research collaborations, further supporting market expansion.
Brazil leads the Latin America inflammatory bowel disease treatment market, exhibiting remarkable growth during the analysis period.
- The growing incidence of both Crohn’s disease plus ulcerative colitis has heightened the demand for more effective treatment options especially for the younger population. Brazil does have a large patient pool, and also people better understand gastrointestinal health, and this accelerates early diagnosis, and starts therapies sooner, and it fuels market growth.
- Access to biologics and targeted therapies is improving because of broader insurance coverage and government investments to strengthen public healthcare under the Unified Health System (SUS) which were previously limited to private healthcare settings.
- Leading multinational pharmaceutical companies coupled with continuing clinical trials in Brazil are fostering the introduction toward revolutionary biologics plus biosimilars. IBD patients can have more treatment options now.
Saudi Arabia market to experience substantial growth in the Middle East and Africa inflammatory bowel disease treatment industry in 2024.
- Rising prevalence of Crohn’s disease and ulcerative colitis, largely linked to changing dietary habits, sedentary lifestyles, and growing urbanization, has significantly increased the patient population requiring effective treatment options. The country’s strong healthcare infrastructure, coupled with heavy government investments under the Vision 2030 initiative, is fostering greater access to advanced biologic therapies and targeted treatments for IBD.
- Increasing awareness among patients and healthcare professionals about early diagnosis and disease management is further accelerating treatment adoption. Moreover, collaborations between global pharmaceutical companies and Saudi healthcare providers are enabling the introduction of innovative biologics and biosimilars that improve patient outcomes and reduce overall treatment costs.
- The government’s commitment to expanding specialized gastroenterology centers and research initiatives is also fueling the availability of clinical expertise and modern therapeutic options.
- Additionally, rising healthcare expenditure, coupled with improved insurance coverage for chronic disease management, supports wider adoption of high-cost biologics.
Inflammatory Bowel Disease Treatment Market Share
global inflammatory bowel disease treatment industry presents a competitive in nature landscape, characterized by the presence of both global leaders and numerous regional players. Key players such as Takeda, AbbVie Inc., Pfizer, UCB, and Johnson & Johnson collectively account for 81.4% of the total market share, leveraging their strong product portfolios, global distribution networks, and focus on technological integration. These companies dominate through continuous innovation in biologics and targeted therapies, strategic collaborations with healthcare providers, and aggressive expansion into emerging markets, reinforcing their leadership in both clinical efficacy and commercial reach.
Beyond the dominant global leaders, the IBD treatment market features a diverse array of mid-sized and regional companies such as Boehringer Ingelheim, UCB, and Biogen. These players cater to specific therapeutic niches, including biosimilars, cost-effective generics, and regionally approved formulations for Crohn’s disease and ulcerative colitis. Their competitive strength lies in localized manufacturing, agile regulatory navigation, and pricing strategies tailored to public healthcare systems. With rising demand for affordable biologics, patient-centric delivery formats, and expanded access in emerging economies, many of these companies are scaling up through strategic licensing deals, regional partnerships, and customized product offerings.
Inflammatory Bowel Disease Treatment Market Companies
Few of the prominent players operating in the inflammatory bowel disease treatment industry include:
- AbbVie
- Alvotech
- Amgen
- Biogen
- Boehringer Ingelheim
- Celltrion
- Dr Falk Pharma
- Eli Lilly
- Ferring
- Johnson & Johnson
- ORGANON
- Pfizer
- Takeda Pharmaceuticals
- UCB
- AbbVie
AbbVie leads the inflammatory bowel disease treatment market with a share of 27.8% in 2024. AbbVie Inc. is focused on the discovery, development, manufacturing, and commercialization of advanced therapies for complex and chronic health conditions. The company's diversified product portfolio includes treatments across key therapeutic areas such as immunology, oncology, neuroscience, eye care, virology, and aesthetics. AbbVie leverages a broad network of distributors, healthcare providers, and strategic alliances to market its products. It maintains a strong intellectual property position through an extensive portfolio of issued patents and a robust pipeline, supported by proprietary research and licensing agreements. Established as a corporate spin-off from Abbott Laboratories, AbbVie operates as an independent entity.
Amgen is engaged in discovering, developing, manufacturing, and marketing biotechnology-based therapies aimed at treating serious illnesses. The product portfolio of the company comprises medicines for oncology, inflammation, cardiovascular disease, bone health and rare diseases. The company collaborates with healthcare professionals to advance biologic science using tools such as human genetic data and AI-driven platforms.
UCB is engaged in discovering, developing, manufacturing, and marketing biopharmaceutical solutions for people living with severe diseases. The product portfolio of the company comprises treatments for neurological and autoimmune disorders, including epilepsy, Parkinson’s disease, and chronic inflammatory conditions.
Inflammatory Bowel Disease Treatment Industry News
- In May 2025, Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd., and Alvotech announced that the U.S. Food and Drug Administration (FDA) had approved SELARSDI (ustekinumab-aekn) injection as interchangeable with the reference biologic Stelara (ustekinumab). This approval may help the company acquire an enhanced customer base.
- In July 2023, Boehringer Ingelheim announced that the U.S. Food and Drug Administration (FDA) had approved the Cyltezo Pen, a new autoinjector option for Cyltezo (adalimumab-adbm), an FDA-approved Interchangeable biosimilar to Humira (adalimumab). Initially approved as a pre-filled syringe, Cyltezo is indicated to treat multiple chronic inflammatory diseases. This approval helped the company to generate enhanced business revenue.
The inflammatory bowel disease treatment market research report includes an in-depth coverage of the industry with estimates and forecasts in terms of revenue in USD Million from 2021 - 2034 for the following segments:
Market, By Treatment Type
- Crohn's disease
- Ulcerative colitis
Market, By Drug Class
- First-line treatment
- Aminosalicylates
- Corticosteroids
- Second-line treatment
- IL inhibitors
- TNF inhibitors
- JAK inhibitors
- Anti-integrin
- S1P receptor modulator
- Combination therapy
- TNF inhibitors + thiopurines
- Other combination therapies
Market, By Route of Administration
- Injectable
- Oral
- Rectal
Market, By Distribution Channel
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
What was the valuation of the injectable route of administration segment in 2024?
Injectables accounted for USD 22.4 billion in 2024, dominating the market due to widespread use of biologics in moderate-to-severe IBD.
What is the growth outlook for oral administration from 2025 to 2034?
Oral therapies are projected to grow at a 6.7% CAGR through 2034.
Which region leads the inflammatory bowel disease treatment market?
North America held 65.7% share in 2024. Growth is driven by high prevalence of IBD, strong adoption of biologics, and robust reimbursement frameworks.
What are the upcoming trends in the inflammatory bowel disease treatment industry?
Key trends include rising adoption of biologics and biosimilars, technological advances in drug delivery (subcutaneous injectors, oral small molecules), and precision medicine approaches using biomarkers and genetics.
Who are the key players in the inflammatory bowel disease treatment market?
Key players include AbbVie, Takeda, Johnson & Johnson, Pfizer, UCB, Amgen, Biogen, Boehringer Ingelheim, Celltrion, and Eli Lilly
What is the market size of the inflammatory bowel disease treatment industry in 2024?
The market size was USD 26.6 billion in 2024, with a CAGR of 5.9% expected through 2034 driven by rising IBD prevalence, favorable reimbursement, and growing awareness of early diagnosis.
What is the current inflammatory bowel disease treatment market size in 2025?
The market size is projected to reach USD 28.1 billion in 2025.
How much revenue did the Crohn’s disease segment generate in 2024?
Crohn’s disease treatment leading the market with a 60.4% share.
What is the projected value of the inflammatory bowel disease treatment market by 2034?
The IBD treatment market is expected to reach USD 47.3 billion by 2034, supported by adoption of biologics, targeted therapies, and expansion in emerging markets.
Inflammatory Bowel Disease Treatment Market Scope
Related Reports


