Summary
Table of Content

HSV Testing Market
Get a free sample of this report
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
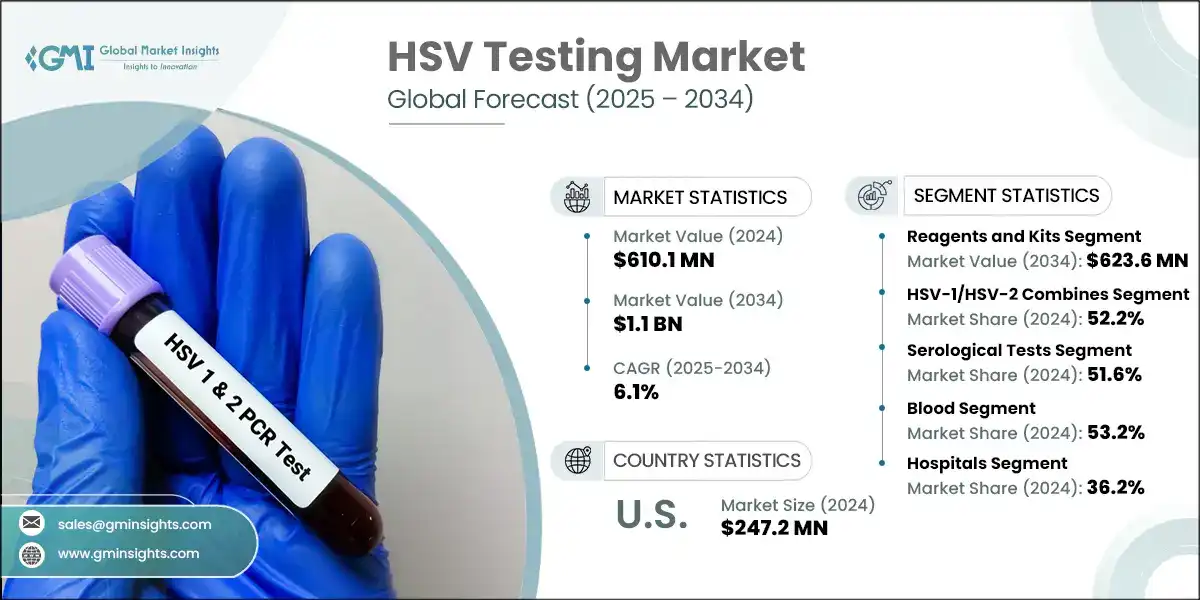
HSV Testing Market Size
The global HSV testing market was valued at USD 610.1 million in 2024 and is projected to grow from USD 645.2 million in 2025 to USD 1.1 billion by 2034, expanding at a CAGR of 6.1%. The market has experienced consistent growth, driven by the rising prevalence of HSV infections globally. Increased awareness of sexually transmitted infections (STIs) and the importance of early diagnosis are key factors boosting demand for HSV testing across clinical and home-care settings.
To get key market trends
The adoption of advanced diagnostic technologies, such as multiplex PCR assays, point-of-care testing, rapid testing kits, and automated serology platforms, has significantly improved diagnostic accuracy and accessibility. These innovations are particularly beneficial in resource-limited settings and high-throughput systems. Government-led sexual health awareness campaigns and expanded screening programs further contribute to market growth.
Additionally, the integration of HSV testing into broader STI panels and electronic health platforms enhances diagnostic reach and efficiency in both developed and emerging markets. The HSV testing market focuses on detecting and monitoring herpes simplex virus infections through molecular and serological tests conducted in clinical and point-of-care laboratories. Major players in this market include Abbott Laboratories, Bio-Rad Laboratories, Becton, Dickinson and Company, and F. Hoffmann-La Roche.
The market value increased from USD 517.8 million in 2021 to USD 577.4 million in 2023. This growth is primarily attributed to the rising global burden of HSV infections and the growing demand for accurate and early diagnostic solutions. The adoption of molecular and serological testing has been supported by public health initiatives, technological advancements, and heightened awareness of STIs. Furthermore, the healthcare sector's shift toward decentralized and accessible diagnostics has driven the preference for rapid and point-of-care HSV tests, particularly in outpatient and home-care settings. These solutions improve patient comfort, reduce stigma, and enable timely clinical decision-making.
The surge in HSV infections is one of the key factors aiding the growth of the HSV treatment (Market size was around USD 2.6 billion in 2022 and is poised to expand at 4% CAGR from 2023 to 2032), testing industry, as early detection is critical for managing symptoms and preventing transmission. As reported by the World Health Organization (WHO) in 2025, an estimated 3.8 billion people under age 50 (64%) globally have HSV-1, the main cause of oral herpes, while 520 million people aged 15–49 (13%) have HSV-2, the primary cause of genital herpes. These alarming statistics underscore the urgent need for advanced, scalable, and accessible HSV diagnostic tools, which are increasingly being adopted by hospitals, diagnostic laboratories, and home-care providers worldwide.
The increasing prevalence of HSV infections is a significant driver of growth in the HSV treatment and testing market. Early detection remains critical for managing symptoms and preventing transmission. According to the World Health Organization (WHO), by 2025, an estimated 3.8 billion individuals under the age of 50 (64% of the global population) are expected to have HSV-1, the primary cause of oral herpes, while 520 million individuals aged 15-49 (13%) are projected to have HSV-2, the main cause of genital herpes. These statistics highlight the urgent need for advanced, scalable, and accessible HSV diagnostic tools, which are increasingly being adopted by hospitals, diagnostic laboratories, and home-care providers globally.
Additionally, government initiatives and funding for STI surveillance and prevention programs are significantly supporting the expansion of HSV testing. For instance, the CDC’s Strengthening STD Prevention and Control for Health Departments (STD PCHD) program provides funding to state and local health departments to monitor STI cases, promote testing and treatment best practices, and reduce STI-related health disparities. These efforts are particularly focused on high-risk populations such as adolescents, young adults, and pregnant women, and are helping to drive demand for reliable HSV diagnostics.
HSV testing solutions are an integral part of modern infectious disease diagnostics. They include specialized molecular and serological platforms designed to detect herpes simplex virus types 1 and 2. These systems utilize advanced technologies such as PCR, and rapid point-of-care kits to identify viral DNA or antibodies with high sensitivity and specificity. HSV testing tools are essential for early detection, clinical management, and epidemiological surveillance, offering features such as multiplexing, automation, and compatibility with decentralized testing environments.
HSV Testing Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 610.1 Million |
| Forecast Period 2025 – 2034 CAGR | 6.1% |
| Market Size in 2034 | USD 1.1 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising prevalence of HSV infections | The rising incidence of HSV infections is increasing the demand for accurate diagnostic tools across clinical and home-care settings. |
| Ongoing advancements in molecular diagnostic technologies | The HSV diagnostics market is witnessing technological advancements in PCR and multiplex platforms to improve testing sensitivity and speed. |
| Government initiatives and funding for STI surveillance and prevention programs | Government healthcare initiatives and awareness programs are driving HSV testing adoption, particularly in high-risk population segments. |
| Pitfalls & Challenges | Impact |
| Regulatory and reimbursement challenges | The HSV testing vendors face significant regulatory hurdles across different regions, with varying approval processes and compliance requirements. |
| Social stigma and privacy concerns | The market expansion faces challenges in conservative and underserved regions due to existing cultural barriers that affect diagnostic test acceptance. |
| Opportunities: | Impact |
| Ongoing enhancement in home-based and self-testing kits | Enables discreet, accessible, and scalable testing, especially for asymptomatic individuals and remote populations. |
| Market Leaders (2024) | |
| Market Leaders |
21.5% Market share |
| Top Players |
Collective market share in 2024 is 61% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Countries | India, Brazil, Mexico, and South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
HSV Testing Market Trends
- The worldwide market is evolving rapidly, shaped by both macro- and micro-level trends. At the macro level, rising global prevalence of HSV infections is driving demand for reliable and scalable diagnostic platforms. According to the WHO, 205 million people aged 15-49 (5.3%) experienced at least one symptomatic episode of genital herpes in 2020, underscoring the need for early detection and clinical management.
- At the micro level, ongoing advancements in molecular diagnostics, including multiplex PCR platforms, automated serological assays, and rapid point-of-care kits, are improving diagnostic speed, sensitivity, and accessibility. These technologies are increasingly integrated into outpatient clinics, emergency care, and home-based testing environments, enabling timely intervention and reducing transmission risks.
- Moreover, government initiatives and funding for STI surveillance and prevention programs, such as the CDC's STD PCHD program updated in January 2025 are expanding testing coverage and public awareness. These efforts are particularly focused on high-risk populations and underserved regions, helping to reduce stigma and improve health outcomes.
- The market is also witnessing a shift toward digital and decentralized diagnostics, with manufacturers focusing on user-friendly interfaces. These features support remote collaboration, personalized care, and scalable deployment across diverse clinical settings. Also, HSV-2 infection increases the risk of acquiring and transmitting HIV, making HSV testing a critical component of broader sexual health strategies.
- As healthcare systems prioritize early detection and prevention, HSV testing platforms are transforming into intelligent diagnostic hubs that support public health goals and individual patient needs.
HSV Testing Market Analysis
Learn more about the key segments shaping this market
The global market was valued at USD 517.8 million in 2021. The market size reached USD 577.4 million in 2023, from USD 546.7 million in 2022.
Based on the product, the HSV testing market is segmented into instruments, and reagents and kits. The reagents and kits segment led this market in 2024, accounting for the highest market share because reagents and kits offer cost-effective, rapid, and easy-to-use solutions, making them ideal for high-throughput testing and routine screening. This segment was valued at USD 340.3 million in 2024 and is projected to reach USD 623.6 million by 2034, growing at a CAGR of 6.3%. This growth can be attributed to their wide use across both centralized laboratories and decentralized settings, including outpatient clinics and point-of-care environments.
In comparison, the instrument segment, valued at USD 269.8 million in 2024, is expected to grow to USD 478.9 million by 2034, with a CAGR of 5.9%, supported by increasing demand for automated and high-throughput diagnostic platforms in clinical laboratories and hospitals, which improve accuracy and reduce manual errors.
- Reagents and kits dominate the HSV testing market due to their widespread use in both centralized laboratories and decentralized care settings. These products offer rapid, cost-effective, and user-friendly solutions for detecting HSV-1 and HSV-2, making them ideal for routine screening, outbreak surveillance, and point-of-care diagnostics.
- A major driver for this segment is its flexibility and scalability. Reagents and kits support various testing formats, including PCR-based molecular assays, serological tests, and rapid lateral flow devices. Their compatibility with automated platforms and minimal sample preparation requirements significantly streamlines workflows, reduces turnaround times, and improves clinical decision-making.
- For example, Abbott's Alinity m HSV/VZV Assay is a high-throughput molecular test that delivers automated sample-to-answer results, enhancing lab efficiency and diagnostic accuracy. It is designed for use in large-scale screening programs and integrates seamlessly with broader STI panels.
- Similarly, Bio-Rad's BioPlex 2200 HSV-1 & HSV-2 IgG Assay offers multiplex serological testing with high sensitivity and specificity. It enables simultaneous detection of HSV antibodies and is widely used in clinical laboratories for differentiating between HSV-1 and HSV-2 infections.
- Overall, the reagents and kits segment is poised to remain the cornerstone of HSV diagnostics, driven by innovation in assay design, growing demand for home-based testing, and global efforts to improve STI surveillance and control.
Based on the type, the HSV testing market is segmented into HSV-1/HSV-2 combines, HSV-1, and HSV-2. The HSV-1/HSV-2 combines segment accounted for the highest market share of 52.2% in 2024.
- These dual-target molecular and serological assays are designed to detect both types of herpes simplex virus simultaneously, offering a comprehensive diagnostic solution that simplifies clinical workflows and enhances screening efficiency.
- These dual-target molecular tests are commonly used in clinical and public health settings due to their efficiency, diagnostic accuracy, and ability to inform tailored treatment strategies. The growing need to identify asymptomatic carriers and make informed prenatal decisions has further bolstered the adoption of combined kits, especially in high-risk and reproductive health contexts.
- A major driver for this segment is its ability to streamline testing procedures, particularly in high-throughput environments such as hospitals, diagnostic laboratories, and public health programs. Combined assays reduce the need for multiple tests, minimize sample handling, and enable faster clinical decision-making.
- Conversely, the HSV-2 segment is expected to expand at the highest CAGR of 6.4%. The rising global prevalence of HSV-2, particularly in sexually active populations, is driving the need for more targeted and sensitive diagnostic tools. HSV-2 is the primary cause of genital herpes, which often goes undiagnosed due to asymptomatic cases.
Based on test type, the HSV testing market is segmented into serological tests, point-of-care tests, and direct detection tests. The serological tests segment accounted for the highest market share of 51.6% in 2024.
- These tests are particularly valued for their ability to detect HSV antibodies, making them highly effective in identifying past exposure and asymptomatic infections. Their broad application spans population-level surveillance, prenatal screening, and routine STI diagnostics.
- These tests are well-suited for high-throughput laboratory environments, offering automated result interpretation, batch processing, and compatibility with centralized diagnostic workflows. Their affordability and scalability make them ideal for public health programs, hospitals, and diagnostic labs.
- However, despite their dominance, concerns persist regarding sensitivity in early-stage infections and the inability to distinguish between active and latent infections. As molecular methods become more accessible, there is growing interest in transitioning to tests that offer higher accuracy and faster turnaround times.
- Nevertheless, serological assays remain the backbone of HSV diagnostics, supported by their cost-effectiveness, ease of implementation, and integration with STI panels. Their continued use is expected in screening programs, epidemiological studies, and clinical decision-making, especially in resource-limited settings.
Based on the sample type, the HSV testing market is segmented into blood, swabs, cerebrospinal fluid, and urine. The blood segment accounted for the highest market share of 53.2% in 2024.
- Blood-based diagnostics are preferred due to their high accuracy, broad clinical acceptance, and ability to detect both HSV-1 and HSV-2 antibodies reliably.
- Blood samples are widely used in hospital laboratories, prenatal screening programs, and routine sexual health assessments, where precision and comprehensive STI screening are essential. Their reliability in identifying past exposure and asymptomatic infections has made them the preferred choice for serological testing.
- Advancements in assay design, such as high-sensitivity ELISA and chemiluminescence-based tests, have improved result precision and reduced processing time, making blood-based diagnostics more efficient and scalable.
- Additionally, blood-based tests are often integrated into comprehensive STI panels, offering efficiency in multi-pathogen detection and compatibility with high-throughput testing platforms. This makes them ideal for public health programs and centralized diagnostic labs.
- Despite the emergence of non-invasive alternatives such as swabs and urine samples, the diagnostic reliability and workflow compatibility of blood samples continue to position them as the primary sample type across clinical and public health settings.
Learn more about the key segments shaping this market
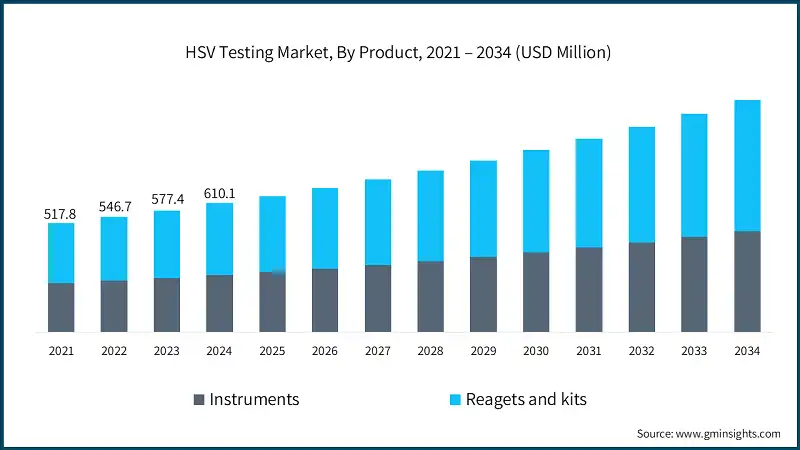
Based on end use, the HSV testing market is segmented into hospitals, diagnostic laboratories, home care/self-testing, and other end users. The hospitals segment accounted for the highest market share of 36.2% in 2024.
- Hospitals serve as the central hubs for sexually transmitted infection (STI) screening, including herpes simplex virus (HSV) types 1 and 2, making them key users of both traditional and advanced molecular diagnostic platforms.
- This segment benefits from high patient throughput, government-backed STI screening initiatives, and integrated care delivery models, which collectively support the adoption of comprehensive HSV testing solutions. Hospitals typically utilize multiplex PCR systems, serological assays, and rapid antigen tests for timely and accurate diagnosis, especially in emergency and prenatal care settings.
- Moreover, hospitals have extensive diagnostic infrastructure and the availability of trained clinical personnel, which enables them to perform accurate and timely HSV testing, manage complex cases efficiently, and support integrated care pathways for patients with sexually transmitted infections.
- Additionally, the home care/self-testing segment, accounting for 19.7% market share in 2024, is expanding rapidly due to growing consumer demand for privacy, convenience, and faster results. This segment is driven by increased awareness of sexually transmitted infections, rising internet-based health education, and the availability of FDA-approved and CE-marked self-testing kits.
Looking for region specific data?
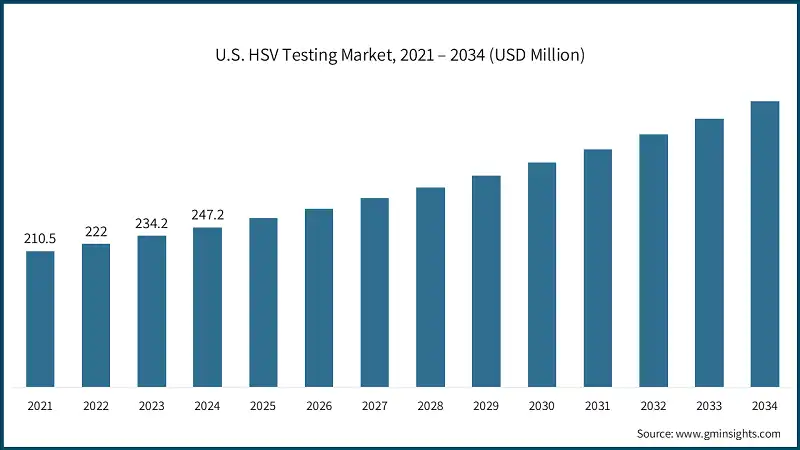
North America dominated the global HSV testing market with the highest market share of 42.5% in 2024. The region has advanced healthcare infrastructure, and the rate of adoption of innovative medical technologies is also high.
- The U.S. market was valued at 210.5 million and USD 222 million in 2021 and 2022, respectively. In 2024 the market size reached USD 247.2 million from USD 234.2 million in 2023.
- Herpes simplex virus (HSV) infections are highly prevalent in the U.S., making the country a key contributor to the global HSV testing market. More than 50% of the adult population is affected by oral herpes (commonly known as cold sores or fever blisters), with most individuals contracting the virus during childhood through casual contact such as kissing.
- Genital herpes remains a common sexually transmitted infection in the U.S. According to the Centers for Disease Control and Prevention (CDC), there were approximately 572,000 new genital herpes infections among individuals aged 14 to 49 in 2018. This data, reaffirmed in CDC’s 2024 clinical updates, highlights the persistent burden of HSV in the country and underscores the need for widespread and accessible testing solutions.
- Additionally, about 12% of individuals aged 14-49 have genital HSV-2 infections, yet up to 90% remain unaware of their status due to asymptomatic or mild presentations, as reported by the American Sexual Health Organization (ASHO). These statistics underscore the critical need for accurate, discreet, and accessible testing platforms across clinical and home-based settings.
- Moreover, the process of regulatory approvals is regularized in developed countries such as the U.S., and the country also has high public awareness and robust R&D investment, which support the continuous rollout of innovative solutions.
- Thus, the market is expected to see sustained revenue growth through 2034, driven by both public health initiatives and private sector innovation.
Europe HSV testing market accounted for USD 164.4 million in 2024 and is anticipated to show lucrative growth over the forecast period.
- Europe is seeing a surge in the number of cases of sexually transmitted infections, as reported by the European Centre for Disease Prevention and Control (ECDC). According to the latest ECDC annual epidemiologic reports on sexually transmitted infections, the notification rate for confirmed gonorrhea cases in European Union/European Economic Area countries increased by 31% in 2023 compared to 2022 and has increased by 321% since 2014. As a result, the awareness towards sexually transmitted infections (STIs) such as HSV is increasing in Europe.
- Countries such as Germany, France, and the UK are leading in the adoption of advanced HSV diagnostic platforms, particularly within public healthcare systems that emphasize preventive care and prenatal screening. These nations are integrating molecular assays, multiplex PCR platforms, and serological testing into routine STI screening programs, improving diagnostic accuracy and accessibility.
- With continued investment in public-private partnerships, mobile screening initiatives, and AI-enabled diagnostic platforms, the European HSV testing market is expected to maintain strong momentum through 2034.
Germany market is projected to experience steady growth between 2025 and 2034.
- The HSV testing market in Germany is expanding steadily, supported by strong healthcare infrastructure, rising public awareness, and increasing prevalence of herpes simplex virus infections. According to the National Institutes of Health (NIH) reported in January 2025, 10 to 15 out of every 100 people in Germany carry HSV viruses in their body, and 10 to 30% of them develop genital herpes, with the condition being somewhat more common in women than in men. These figures highlight the need for widespread and accessible diagnostic solutions across clinical and outpatient settings.
- Germany's market growth is further driven by national sexual health programs, routine STI screening, and the integration of molecular and serological HSV testing platforms in hospitals and diagnostic laboratories. The country is also investing in digital diagnostics, remote testing kits, and AI-enabled platforms to improve detection rates and reduce transmission.
- Moreover, Germany's healthcare system supports early diagnosis and preventive care, which aligns with broader European efforts to reduce the burden of STIs. Public-private partnerships and mobile screening initiatives are helping expand access to testing.
The Asia Pacific region is projected to be valued at USD 143.9 million in 2025 and is expected to reach USD 260 million by 2034.
- The Asia Pacific HSV testing market is projected to grow at the fastest CAGR over the forecast period, driven by rising awareness of sexually transmitted infections, expanding government-sponsored health programs, and increasing disposable incomes across emerging economies.
- A key growth factor is the region's rapidly aging population, which is associated with increased vulnerability to infections and a higher demand for diagnostic services.
- In Asia Pacific, the number of older persons is projected to increase from 630 million in 2020 to about 1.3 billion by 2050. By 2050, one quarter of the population will be 60 or older. This demographic shift is anticipated to significantly boost demand for HSV testing, particularly in hospital and outpatient settings.
Japan HSV testing market is poised to witness lucrative growth between 2025-2034.
- The country's aging demographic is a major driver, with 29.1% of the population aged 65 and older as of 2023, according to Statista. This demographic trend is increasing demand for early detection, preventive care, and routine STI screening, particularly among older adults who may be more susceptible to viral reactivation and complications.
- Government initiatives and public awareness campaigns are expanding access to screening services, positioning Japan as a key player in the Asia Pacific imaging landscape.
Brazil is experiencing significant growth in the HSV testing market.
- The HSV testing market in Brazil is witnessing steady growth, driven by rising public health awareness, demographic shifts, and expanding access to diagnostic services. Genital herpes is among the most common sexually transmitted infections in the country, with increasing demand for non-invasive, accurate, and accessible testing platforms across both urban and rural regions.
- Brazil's aging population is contributing to increased screening needs. As per the Brazilian Institute of Geography and Statistics, the number of individuals aged 65 and older reached 22.17 million in 2022, accounting for 10.9% of the total population, a 57.4% increase since 2010.
- With continued investment in digital health infrastructure, public-private partnerships, and education campaigns, Brazil is emerging as a key market in Latin America for HSV testing, with strong growth projected through 2034.
The HSV testing market in Saudi Arabia is expected to experience significant and promising growth from 2025 to 2034.
- The market in Saudi Arabia is expected to grow steadily through 2034, driven by demographic shifts, rising public health awareness, and expanding access to diagnostic services. One of the most significant growth drivers is the country's rapidly aging population.
- Saudi Arabia's elderly population is rising at a much faster pace than any other segment. It is estimated that the population aged 60 years and above will grow from 2 million (5.9 percent of the total population) in 2020 to 10.5 million by 2050. This demographic transition is expected to increase demand for preventive healthcare, including routine STI screening and early detection of HSV infections.
HSV Testing Market Share
- The top 5 players, such as Abbott Laboratories, F. Hoffmann-La Roche, Becton, Dickinson and Company, Bio-Rad Laboratories, and Thermo Fisher Scientific, collectively held 61% of the total market share. These companies maintain their leadership through a combination of technological innovation, regulatory approvals, AI integration, and strategic collaborations with healthcare providers and public health agencies.
- Abbott Laboratories leads the market with its molecular diagnostic platforms, including real-time PCR systems and multiplex assays that offer high sensitivity and specificity for HSV-1 and HSV-2 detection. Abbott's solutions are widely adopted in hospitals, reference laboratories, and national screening programs due to their automation capabilities, scalability, and integration with digital health systems.
- Meanwhile, emerging players such as CTK Biotech, TRUPCR, ZEUS Scientific, AdvaCare Pharma, and bioWORLD are gaining traction by introducing cost-effective, rapid, and AI-enabled HSV testing kits. These companies are addressing unmet needs in point-of-care diagnostics, telehealth-compatible platforms, and low-resource settings, helping expand access to testing in underserved regions.
- To increase market share, firms are introducing cost-effective multimodal diagnostic platforms that combine serological and molecular HSV testing, often enhanced by artificial intelligence for improved result interpretation and workflow automation. Advances in sample-to-answer systems, automated quality control, and real-time analytics are transforming the competitive landscape, enabling faster diagnosis, better clinical decision-making, and broader accessibility across both centralized labs and point-of-care settings.
HSV Testing Market Companies
Few of the prominent players operating in the HSV testing industry include:
- Abbott Laboratories
- AdvaCare Pharma
- Becton, Dickinson and Company
- Bio-Rad Laboratories
- bioWORLD
- CTK Biotech
- DiaSorin
- F. Hoffmann-La Roche
- Hologic
- Innermost Healthcare
- McKesson Medical-Surgical
- Meridian Bioscience
- PrivaPath Diagnostics
- Quest Diagnostics
- QuidelOrtho Corporation
- Thermo Fisher Scientific
- TRUPCR
- ZEUS Scientific
- Bio-Rad Laboratories
Bio-Rad stands out in the HSV testing market with its serological assays for detecting IgG and IgM antibodies to HSV-1 and HSV-2. These tests are widely used for screening prior infections, monitoring at-risk pregnancies, and diagnosing neonatal and encephalitic HSV cases. Bio-Rad's platforms are valued for their clinical reliability, ease of use, and integration into routine laboratory workflows, making them a preferred choice for hospitals and reference labs. The company's focus on high-throughput immunoassays and type-specific detection supports better treatment planning and patient follow-up.
- Thermo Fisher Scientific
Thermo Fisher Scientific provides diagnostic tools for herpes simplex virus (HSV) typing, including the IMAGEN and PathoDx kits. These kits utilize direct immunofluorescence assays (DFA) for rapid and sensitive detection and typing of HSV-1 and HSV-2 in cell cultures. Thermo Fisher's strength lies in its broad reagent catalog, validated control slides, and compatibility with automated systems, which streamline testing workflows and reduce turnaround times. The company's commitment to precision diagnostics and lab efficiency positions it as a key player in both centralized and decentralized testing environments.
- Hologic
Hologic's Aptima HSV 1 & 2 assay is a nucleic acid amplification test (NAAT) that provides molecular-level precision in diagnosing genital herpes, recommended by CDC and WHO for genital herpes diagnosis. The assay offers high sensitivity, rapid results, and minimal hands-on time, making it ideal for high-volume labs and urgent care settings. Hologic's emphasis on early detection, workflow automation, and clinical accuracy aligns with its broader mission to improve health outcomes through proactive diagnostics. Its HSV testing solutions are increasingly adopted in women's health programs, prenatal screening, and sexual health clinics.
HSV Testing Industry News:
- In October 2024, Microbix Biosystems Inc. introduced a novel HSV test control at the European Microbiology and Molecular Diagnostics (EMMD) conference. This innovation is designed to enhance the accuracy and reliability of HSV diagnostic assays by providing high-quality, standardized controls for both HSV-1 and HSV-2. The launch is expected to support assay validation, quality assurance, and regulatory compliance across clinical laboratories, reinforcing Microbix's role in advancing molecular diagnostics in the HSV testing space.
- In May 2022, QIAGEN announced the launch of its HSV 1/2 Herpes Assay with CE-IVD certification for use on the NeuMoDx Integrated PCR System. This fully automated molecular diagnostic platform is designed to deliver rapid and accurate HSV-1 and HSV-2 detection. The launch is expected to enhance clinical workflows across European laboratories and strengthen QIAGEN's position in the market by offering scalable, high-throughput solutions for routine STI screening.
The HSV testing market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Product
- Instruments
- Reagents and kits
Market, By Type
- HSV-1/HSV-2 Combines
- HSV-1
- HSV-2
Market, By Test Type
- Serological tests
- Direct detection tests
- PCR
- Viral culture
- Point-of-care tests
Market, By Sample Type
- Blood
- Swabs
- Cerebrospinal fluid
- Urine
Market, By End Use
- Hospitals
- Diagnostic laboratories
- Home care/self-testing
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- Saudi Arabia
- South Africa
- UAE
Frequently Asked Question(FAQ) :
Who are the key players in the HSV testing market?
Key players include Abbott Laboratories, F. Hoffmann-La Roche, Becton, Dickinson and Company, Bio-Rad Laboratories, Thermo Fisher Scientific, Hologic, Quest Diagnostics, and DiaSorin.
What are the upcoming trends in the HSV testing industry?
Trends include home/self-testing kits, AI-enabled diagnostics, multiplex PCR assays, digital health integration, and decentralized care in emerging markets.
Which region leads the HSV testing market?
North America led the market with a 42.5% share in 2024, due to strong healthcare infrastructure and high STI awareness.
What was the valuation of the serological test segment?
The serological tests segment held 51.6% of the market in 2024, making it the largest segment, with broad use in high-throughput labs and public health programs.
What is the market size of the HSV testing market in 2024?
The market size was USD 610.1 million in 2024, with a CAGR of 6.1% expected through 2034, driven by rising HSV infection prevalence and adoption of advanced diagnostic technologies.
How much revenue did the reagents and kits segment generate?
The reagents and kits segment generated USD 340.3 million in 2024, accounting for the highest market share due to its ease of use and widespread application.
What is the projected size of the HSV testing market in 2025?
The market is projected to reach USD 645.2 million in 2025, driven by increased awareness and integration of HSV tests into broader STI panels.
What is the projected value of the HSV testing market by 2034?
The market is expected to reach USD 1.1 billion by 2034, supported by government STI initiatives, home-based testing demand, and technological advancements in diagnostics.
HSV Testing Market Scope
Related Reports


