Summary
Table of Content

Decentralized Clinical Trials Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Decentralized Clinical Trials Market Size
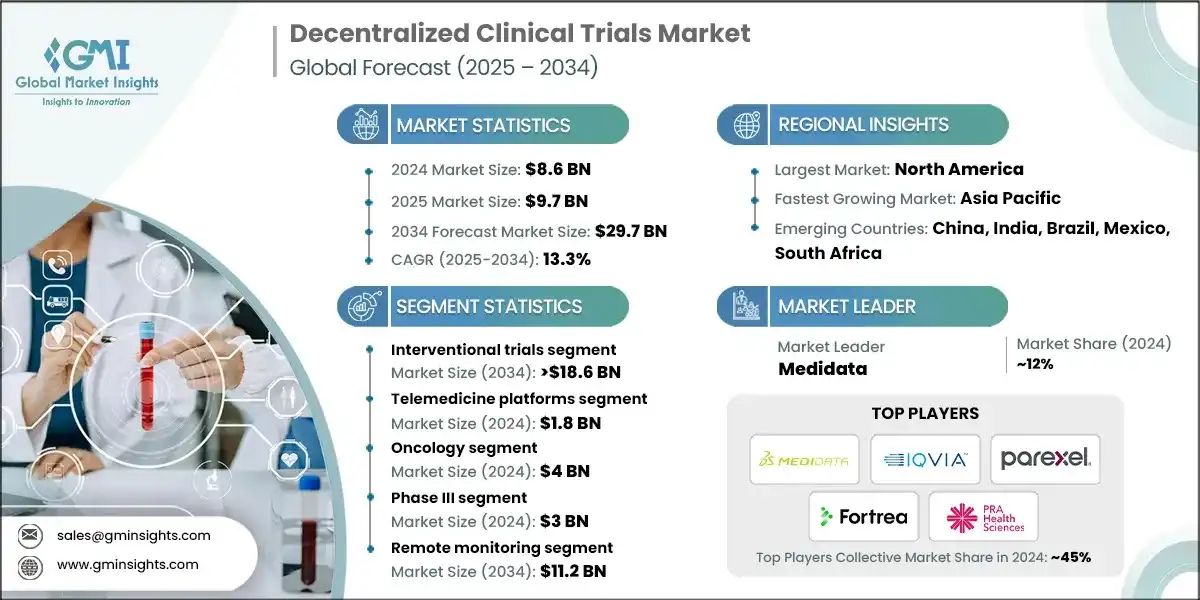
The global decentralized clinical trials market size was valued at USD 8.6 billion in 2024. The market is expected to grow from USD 9.7 billion in 2025 to USD 29.7 billion in 2034, at a CAGR of 13.3% during the forecast period, according to the latest report published by Global Market Insights Inc.
To get key market trends
This growth is attributed to the rise of digital tools such as wearable medical devices, telemedicine platforms, e-consent systems, and electronic patient-reported outcomes (ePRO) that have revolutionized clinical trial operations. Decentralized trials eliminate geographical barriers, allowing patients from remote or underserved regions to participate without traveling to centralized sites. Regulatory authorities worldwide, such as the FDA, Japanese regulatory agencies, and Health Canada, are establishing guidelines for decentralized clinical trials.
For instance, according to FDA data, the number of decentralized trials increased by 50% between 2021 and 2023, with over 1,300 trials incorporating remote elements. The guidelines address remote informed consent procedures, risk-based monitoring approaches, and digital tool validation requirements. In 2023, Health Canada reported that 35% of all clinical trials in the country included at least one decentralized component.
Moreover, decentralized clinical trials reduce operational costs by minimizing physical trial sites, travel expenses, and on-site personnel requirements. According to the FDA, in 2022, approximately 75% of clinical trials incorporated decentralized elements, resulting in a 30% reduction in operational costs. Additionally, remote data collection and virtual visits improve trial efficiency, enabling faster initiation and completion. The NIH reported in 2023 that decentralized trials showed a 40% decrease in patient dropout rates compared to traditional trials. Artificial intelligence analytics and real-time monitoring reduce trial duration through improved patient selection and protocol compliance, with the CDC noting a 25% reduction in trial completion time in 2023.
A decentralized clinical trial (DCT) is a study where some or all trial-related activities take place outside of traditional, central clinical sites, allowing patients to participate from home or local healthcare facilities. This is achieved using telemedicine, digital health technologies like wearables, and direct shipment of study drugs to the patient. Major players in the industry are IQVIA, Medidata, Parexel, Fortrea, and PRA Health Sciences (ICON). These players dominated the market by adopting various strategies such as product expansion and establishing global distribution networks.
Market has witnessed steady growth, growing from USD 5.8 billion in 2021 to USD 7.6 billion in 2023. Governments are also investing in infrastructure and partnerships to facilitate decentralized research, such as the BARDA-Walgreens initiative in the U.S. This institutional backing legitimizes DCTs and accelerates their integration into mainstream clinical research, that further contributes to market growth.
Decentralized Clinical Trials Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 8.6 Billion |
| Market Size in 2025 | USD 9.7 Billion |
| Forecast Period 2025 - 2034 CAGR | 13.3% |
| Market Size in 2034 | USD 29.7 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Technological advancement in digital health | Enables remote data collection, real-time monitoring, and scalable trial execution across diverse geographies. |
| Cost efficiency and accelerated timelines | Enter impact of this driver |
| Regulatory support and framework development | Encourages adoption through clear guidelines for remote protocols, digital consent, and virtual patient engagement. |
| Improved patient accessibility and diversity | Expands trial reach to underserved populations, enhancing recruitment, retention, and data representativeness. |
| Pitfalls & Challenges | Impact |
| Data integrity and standardization | Inconsistent data formats and fragmented systems hinder interoperability, slowing adoption and regulatory acceptance of DCTs. |
| Regulatory complexity | Varying global regulations and unclear compliance frameworks create operational challenges and delay trial approvals across regions. |
| Opportunities: | Impact |
| Integration of AI and wearables | Enables real-time monitoring, predictive analytics, and personalized trial experiences, enhancing efficiency and patient engagement. |
| Expansion into underserved regions | Broadens trial access, improves diversity, and accelerates recruitment by reaching remote and underrepresented populations. |
| Market Leaders (2024) | |
| Market Leaders |
12% market share. |
| Top Players |
Collective market share of ~45% in 2024 |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Decentralized Clinical Trials Market Trends
- Healthcare systems worldwide are embracing models that prioritize patient convenience and accessibility. DCTs align with this shift by enabling remote participation, reducing travel burdens, and improving trial inclusivity. This trend is driven by aging populations, chronic disease prevalence, and demand for personalized medicine.
- In clinical research, it catalyzed the shift from site-centric to decentralized models. Sponsors now prioritize digital-first strategies, making DCTs a permanent fixture in trial planning.
- Moreover, initiatives such as BARDA-Walgreens and government-backed innovation hubs are expanding infrastructure for decentralized trials. These partnerships foster regulatory clarity, technology deployment, and broader patient access, and market growth.
- Cloud-based systems are essential in clinical trial operations, facilitating real-time data collection, remote monitoring, and centralized management. According to the FDA, in 2023, over 75% of clinical trials utilized cloud-based data management systems. Mobile applications are increasingly adopted due to their ease of use, with the NIH reporting a 45% growth in mobile-based trial solutions between 2021-2023.
- Moreover, cloud-based platforms and hybrid app-centric solutions are growing, offering flexible interfaces. Contract Research Organization (CRO) are acquiring digital capabilities, and trial sites are evolving into regional hubs. Patient diversity is improving through remote access and culturally tailored recruitment strategies.
- Additionally, DCTs in 2025 leverage AI for recruitment, anomaly detection, and predictive analytics. Wearables and sensors enable continuous monitoring, while eConsent and telemedicine platforms enhance engagement. Blockchain is emerging for secure data sharing, and AR/VR tools support patient education.
- Unified platforms integrate EDC, CTMS, and eSource systems, streamlining operations. These technologies reduce site burden, improve data quality, and support adaptive trial designs.
- Regulatory bodies like the FDA, EMA, and MHRA have issued updated guidance supporting DCTs. Key changes include alignment with ICH E6 (R3), single IRB models for multicenter trials, and frameworks for AI and real-world data use. Emphasis is placed on data provenance, remote monitoring compliance, and patient privacy.
- These updates streamline approvals, enhance trial diversity, and support hybrid models, making DCTs more scalable and compliant.
- Operationally, DCTs rely on mobile nursing, remote diagnostics, and AI-enhanced logistics. Cloud-native platforms dominate, while hybrid models offer scalable solutions. Training and certification tech ensures protocol adherence and safety. Real-time monitoring and decentralized data capture reduce site dependency, that further drives the growth of the market.
Decentralized Clinical Trials Market Analysis
Learn more about the key segments shaping this market
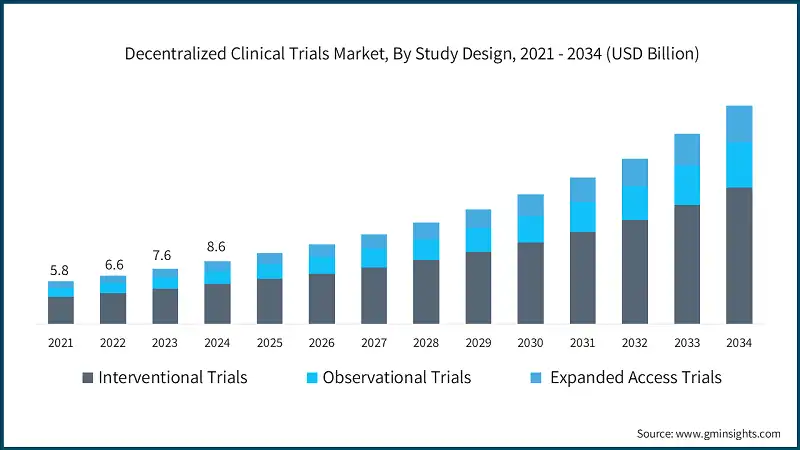
In 2021, the global market was valued at USD 5.8 billion. The following year, it saw an increase to USD 6.6 billion, and by 2023, the market further climbed to USD 7.6 billion.
Based on study design, the global market is divided into interventional trials, observational trials, and expanded access trials. The interventional trials segment accounted for 63.7% of the market in 2024. The segment is expected to exceed USD 18.6 billion by 2034, growing at a CAGR of 13.1% during the forecast period. The interventional trials segment is further divided into randomized controlled trials (RCTs), adaptive clinical trials and pragmatic clinical trials.
- Interventional DCTs reduce the need for hospital visits by enabling remote participation, which improves accessibility for elderly, disabled, and geographically dispersed patients. This patient-centric approach enhances recruitment and retention rates.
- Wearables, mobile apps, ePROs, and telemedicine allow real-time monitoring and data collection, making interventional trials more efficient and scalable. These tools support complex interventions outside traditional clinical settings.
- Regulatory bodies such as the U.S. FDA and EMA are increasingly endorsing remote monitoring and digital tools, making interventional DCTs more viable and compliant. This has encouraged sponsors to adopt decentralized models for interventional studies.
- Further, expanded access trials segment is expected to grow with the fastest CAGR of 14% during the analysis period. Patients with serious or life-threatening conditions are increasingly seeking access to experimental treatments outside traditional trial settings. DCTs enable expanded access by facilitating remote participation and reducing geographic barriers, especially for rare diseases and underserved populations.
- Pharmaceutical companies and CROs are increasingly integrating expanded access into their DCT strategies to improve trial reach, accelerate data collection, and build goodwill with patient communities. This trend is supported by industry alliances and funding initiatives.
Based on technology, the decentralized clinical trials market is segmented into telemedicine platforms, wearable devices, mobile health applications, electronic data capture (EDC) systems, and other technologies. The telemedicine platforms segment dominated the market in 2024 with a revenue of USD 1.8 billion.
- Telemedicine eliminates geographic and mobility barriers, allowing patients from remote or underserved areas to participate in trials. This inclusivity improves recruitment and retention, especially for rare diseases and elderly populations.
- In addition, telemedicine reduces the need for physical trial sites and travel, lowering operational costs and accelerating timelines. Sponsors benefit from streamlined logistics and faster data collection.
- Telemedicine platforms are increasingly integrated with wearables, mobile apps, and ePRO systems, creating a seamless digital infrastructure for decentralized trials. This enhances data capture and trial scalability.
- Telemedicine platforms enable continuous patient monitoring and virtual consultations, improving data quality and protocol adherence. Real-time interactions enhance patient engagement and reduce dropout rates.
Based on the therapeutic area, the decentralized clinical trials market is segmented into oncology, cardiology, neurology, infectious diseases, respiratory disorders and other chronic conditions. The oncology segment dominated the market in 2024 with a revenue of USD 4 billion.
- Cancer remains one of the leading causes of death globally, creating an urgent need for innovative treatments. The high prevalence of oncology cases drives demand for clinical trials, and DCTs offer a scalable way to reach diverse patient populations. Decentralization allows trials to extend beyond specialized hospital sites, enabling broader participation and faster recruitment, especially in underserved regions.
- Oncology trials often require frequent monitoring scans, blood tests, and symptom tracking which traditionally limit participation to specialized centers. DCTs leverage remote monitoring tools, wearables, and telemedicine to collect this data in real time, reducing patient burden and improving retention. This flexibility supports complex trial designs while maintaining data integrity.
- Many cancer patients are willing to participate in trials but face barriers like travel, time, and physical limitations. DCTs remove these barriers by enabling participation from home, improving access and equity. This patient-centric approach enhances engagement, adherence, and outcomes, making oncology trials more inclusive and efficient.
Based on study phase, the decentralized clinical trials market is segmented into phase I, phase II, phase III, and phase IV. The phase III segment dominated the market in 2024 with a revenue of USD 3 billion.
- Phase III trials are the most expensive and complex stage of drug development, often involving thousands of participants across multiple sites. Decentralized models help reduce operational costs by minimizing site visits, streamlining logistics, and enabling remote data collection.
- Phase III trials require large, diverse populations to validate efficacy and safety across demographics. DCTs enable broader geographic reach, including rural and underserved regions, by removing barriers like travel and site access. This inclusivity improves recruitment speed and trial generalizability. Digital platforms and mobile health technologies facilitate remote onboarding and engagement, making it easier to enroll and retain participants globally.
- Additionally, innovation incentives and public-private partnerships are encouraging the use of decentralized models to accelerate drug development timelines. Regulatory flexibility around remote monitoring, eConsent, and telehealth visits has made Phase III trials more adaptable and scalable.
Learn more about the key segments shaping this market
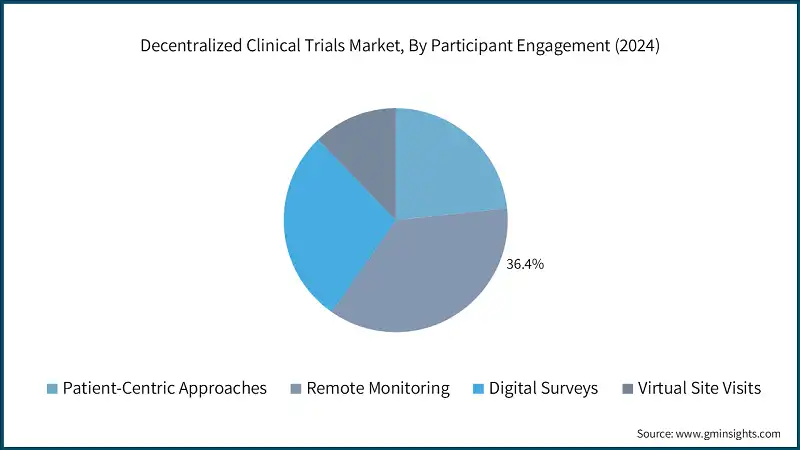
Based on participant engagement, decentralized clinical trials market is classified into patient-centric approaches, remote monitoring, digital surveys, and virtual site visits. The remote monitoring segment dominated the market in 2024 and is expected to reach USD 11.2 billion by 2034.
- Remote monitoring allows participants to engage in trials from their homes, reducing the burden of travel, missed work, and caregiver dependency. This convenience significantly improves retention rates, which are traditionally low in site-based trials due to logistical challenges.
- AI-driven analytics further enhance data quality by identifying anomalies and trends instantly. These capabilities support adaptive trial designs and faster decision-making, making remote monitoring a critical tool for improving trial efficiency and outcomes.
- Advances in cloud computing, cybersecurity, and interoperability have made remote monitoring systems more reliable and compliant. These developments have increased sponsor confidence and investment in decentralized models.
Looking for region specific data?
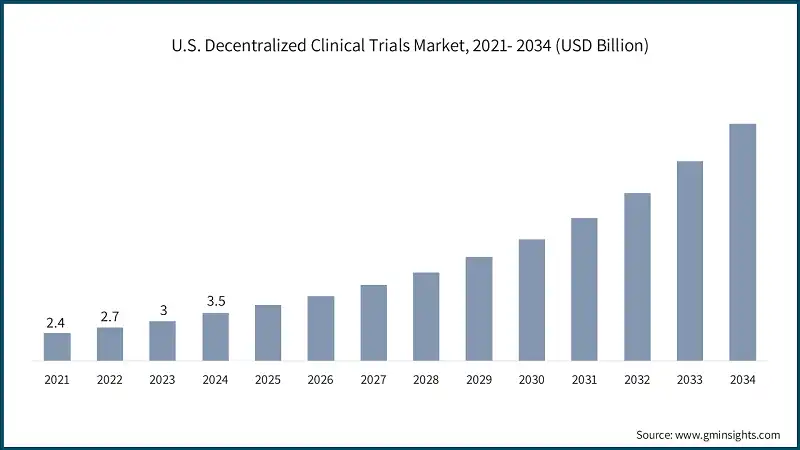
North America Decentralized Clinical Trials Market The North America market dominated the global market with a market share of 44.1% in 2024. The U.S. decentralized clinical trials market was valued at USD 2.4 billion and USD 2.7 billion in 2021 and 2022, respectively. The market size reached USD 3.5 billion in 2024, growing from USD 3 billion in 2023. Europe market accounted for USD 1.7 billion in 2024 and is anticipated to show lucrative growth over the forecast period. Germany dominates the European decentralized clinical trials market, showcasing strong growth potential. The Asia Pacific market is anticipated to grow at the highest CAGR of 13.6% during the analysis timeframe. China decentralized clinical trials market is estimated to grow with a significant CAGR, in the Asia Pacific market. Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period. Saudi Arabia market to experience substantial growth in the Middle East and Africa market in 2024. The market is characterized by a dynamic and moderately consolidated competitive landscape, led by a mix of established life sciences companies and emerging technology innovators. Industry leaders such as IQVIA, Medidata, Parexel, Fortrea, and PRA Health Sciences (ICON) collectively hold around 45% of the global market share. Their dominance is driven by strategic investments in AI-powered platforms, automated trial workflows, and cloud-based data ecosystems. To strengthen their market positions, these companies are pursuing multi-pronged strategies including mergers and acquisitions, partnerships with hospitals, academic institutions, and tech firms, and expansion into remote monitoring and digital patient engagement tools. Their focus remains on improving data accuracy, enhancing platform interoperability, and supporting global precision medicine initiatives. Meanwhile, emerging players and regional innovators are contributing to market growth through the development of mobile health technologies, remote diagnostics, and edge computing-enabled analytics. These solutions are gaining traction in regions such as Asia-Pacific, Latin America, and the Middle East, where rising investments in healthcare digitization and demand for cost-effective, patient-centric research tools are accelerating adoption. The market continues to evolve amid intensifying competition, increasing regulatory alignment (e.g., FDA and EMA support for decentralized protocols), and a shift toward real-time, home-based care models. Companies are actively adapting their product portfolios to meet growing demand for virtual trial capabilities, remote patient monitoring, and preventive health solutions ensuring sustained innovation and global expansion. Prominent players operating in the decentralized clinical trials industry are as mentioned below: Medidata leads the market with a share of ~12% in 2024. Medidata leads with a unified cloud-based platform integrating eConsent, eCOA, and remote monitoring tools, offering end-to-end digital trial management and real-time data insights to enhance patient engagement and trial efficiency. Parexel combines deep therapeutic expertise with decentralized technologies, offering hybrid trial models, remote patient services, and strategic site partnerships to improve trial accessibility, speed, and data quality. IQVIA leverages its global data network, AI-powered analytics, and virtual trial capabilities to deliver scalable, patient-centric decentralized trials, optimizing recruitment, monitoring, and regulatory compliance across diverse therapeutic areas.Europe Decentralized Clinical Trials Market
Asia Pacific Decentralized Clinical Trials Market
Latin American Decentralized Clinical Trials Market
Middle East and Africa Decentralized Clinical Trials Market
Decentralized Clinical Trials Market Share
Decentralized Clinical Trials Market Companies
Decentralized Clinical Trials Industry News
The decentralized clinical trials market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Billion from 2021 - 2034 for the following segments:
Market, By Study Design
- Interventional trials
- Randomized controlled trials (RCTs)
- Adaptive clinical trials
- Pragmatic clinical trials
- Observational trials
- Cohort studies
- Retrospective studies
- Longitudinal studies
- Expanded access trials
Market, By Technology
- Telemedicine platforms
- Wearable devices
- Mobile health applications
- Electronic data capture (EDC) systems
- Other technologies
Market, By Therapeutic Area
- Oncology
- Cardiology
- Neurology
- Infectious diseases
- Respiratory disorders
- Other chronic conditions
Market, By Study Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Market, By Participant Engagement
- Patient-centric approaches
- Remote monitoring
- Digital surveys
- Virtual site visits
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
What is the market size of the decentralized clinical trials market in 2024?
The global decentralized clinical trials (DCT) market was valued at USD 8.6 billion in 2024, driven by widespread adoption of telemedicine, wearable devices, and eConsent systems that improve patient accessibility and reduce trial costs.
What is the market valuation for 2025?
The market is expected to reach USD 9.7 billion in 2025, driven by broader implementation of hybrid trial models and cloud-based patient monitoring platforms.
What is the projected value of the decentralized clinical trials market by 2034?
The market is projected to reach USD 29.7 billion by 2034, growing at a CAGR of 13.3% from 2025 to 2034, supported by increased regulatory support, integration of AI, and expansion into underserved regions.
Which study design segment led the market in 2024?
The interventional trials segment dominated with a 63.7% share in 2024, due to growing adoption of remote participation models that enhance accessibility and retention rates in large-scale studies.
Which technology segment held the largest share in 2024?
Telemedicine platforms led the market with USD 1.8 billion in revenue in 2024, enabling remote consultations, reducing site dependency, and improving patient engagement.
Which therapeutic area dominated the decentralized clinical trials market in 2024?
Which therapeutic area dominated the decentralized clinical trials market in 2024?
Which study phase contributed the highest revenue in 2024?
The phase III trials segment dominated the market with USD 3 billion in 2024, benefiting from large participant pools, global reach, and cost efficiencies through remote data collection.
Which participant engagement approach led the market in 2024?
Remote monitoring dominated the market in 2024 and is projected to reach USD 11.2 billion by 2034, driven by AI-based analytics and cloud-enabled real-time patient tracking.
Which region leads the decentralized clinical trials industry?
North America led the market with a 44.1% share in 2024, supported by advanced digital infrastructure, regulatory clarity, and strong pharmaceutical R&D activity.
Which region is expected to grow the fastest through 2034?
Asia Pacific is anticipated to grow at a CAGR of 13.6%, driven by rapid digitization in China, India, and South Korea and the growing participation of treatment-naïve populations.
Who are the key players in the decentralized clinical trials market?
Major players include Medidata, IQVIA, Parexel, Fortrea, PRA Health Sciences (ICON), Covance, PPD (Thermo Fisher Scientific), and Veeva Systems, collectively holding around 45% market share in 2024.
Decentralized Clinical Trials Market Scope
Related Reports


