Summary
Table of Content

Biological Sample Collection Kits Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Biological Sample Collection Kits Market Size
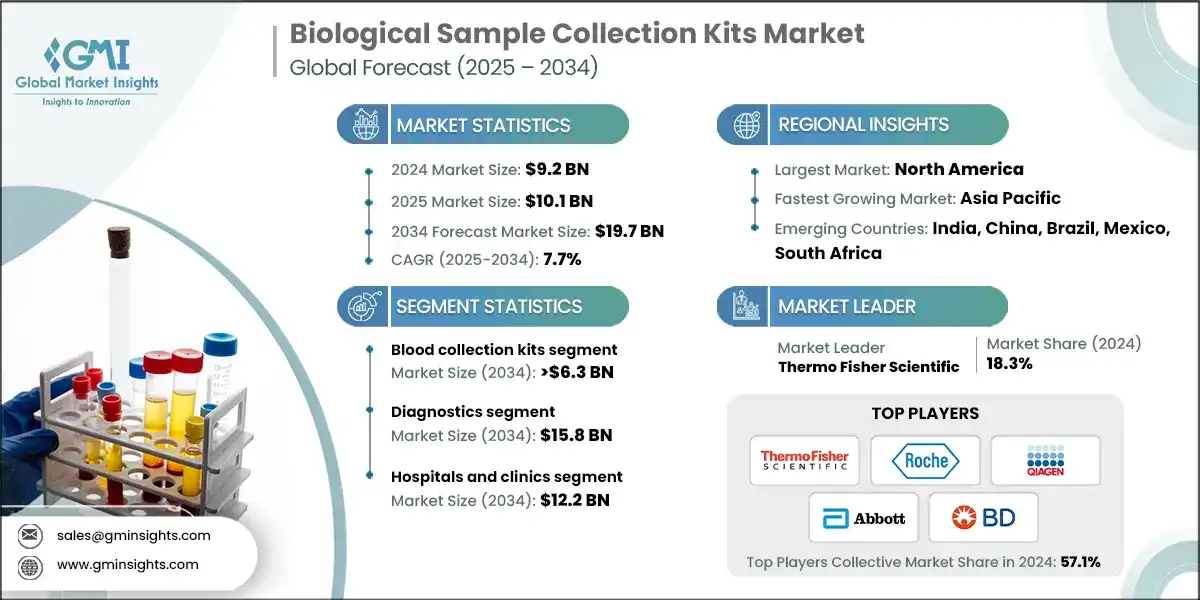
The global biological sample collection kits market was valued at USD 9.2 billion in 2024. The market is expected to reach from USD 10.1 billion in 2025 to USD 19.7 billion in 2034, growing at a CAGR of 7.7% during the forecast period, according to the latest report published by Global Market Insights Inc. Increasing demand for diagnostic testing, growing biobanking, the expanding field of personalized medicine biomarkers, drives market.
To get key market trends
The biological sample collection kits market delivers advanced solutions to diagnostics companies, research laboratories, biopharmaceutical firms, and healthcare providers to enhance sample integrity, diagnostic accuracy, and workflow efficiency. These solutions encompass swabs, urine and blood collection kits, viral transport media, saliva collection systems, and integrated self-sampling platforms that ensure safe, reliable, and contamination-free specimen handling for diagnostics and research applications.
Major players such as Thermo Fisher Scientific, Roche, QIAGEN, Abbott, and Becton, Dickinson and Company (BD) maintain their competitive edge through continuous product innovation, global distribution networks, strategic collaborations, and substantial R&D investments aimed at improving specimen quality, enabling personalized medicine, and meeting the rising demand for decentralized and at-home testing solutions.
The market has increased from USD 6.6 billion in 2021 and reached USD 8.4 billion in 2023. The growth of the biological sample collection kits market is further driven by several evolving healthcare and research trends beyond diagnostic demand and R&D expansion. The rising prevalence of infectious diseases, genetic disorders, and chronic illnesses has accelerated the need for accurate specimen collection systems that support large-scale screening and early disease detection programs. Increasing investments in clinical trials and drug discovery activities are also fueling demand for standardized, contamination-free collection kits that ensure sample stability and reproducibility across study sites.
Moreover, the growing emphasis on precision diagnostics and molecular testing is creating opportunities for specialized sample collection kits compatible with PCR, next-generation sequencing (NGS), and other advanced analytical platforms. The rapid adoption of digital health tools and telemedicine has further expanded the use of home-based sample collection kits, providing convenient, patient-centric solutions that minimize hospital visits. In addition, the global expansion of biopharmaceutical manufacturing and regulatory focus on sample traceability are driving innovation in barcoded, tamper-proof, and temperature-controlled collection systems.
The integration of automation in laboratories and the demand for customized sample collection solutions tailored to specific specimen types such as saliva, plasma, and tissue are further propelling market adoption. Environmental sustainability initiatives are also encouraging the development of eco-friendly and single-use collection materials. Collectively, these factors are strengthening the market’s outlook, as stakeholders continue to prioritize efficiency, reliability, and compliance in specimen handling to improve diagnostic precision, research reproducibility, and clinical outcomes.
Biological sample collection kits are standardized systems designed for the safe, sterile, and efficient collection, preservation, and transport of biological specimens such as blood, saliva, urine, and swabs. These kits maintain sample integrity for accurate diagnostic testing, research analysis, and biobanking applications across healthcare and life science settings.
Biological Sample Collection Kits Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 9.2 Billion |
| Market Size in 2025 | USD 10.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 7.7% |
| Market Size in 2034 | USD 19.7 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing number of laboratory tests | Rising laboratory test volumes are driving demand for standardized biological sample collection kits, improving sample accuracy, efficiency, and enabling laboratories to handle growing diagnostic workloads reliably. |
| Rising usage of lab tests for precise disease diagnosis | Greater reliance on lab-based diagnostics for accurate disease detection is fueling the need for high-quality collection kits, ensuring integrity, reducing errors, and supporting better clinical outcomes. |
| Growing R&D activities and technological advancements in sample collection techniques | Innovations in swabs, transport media, and automated collection methods enhance sample quality and usability, stimulating market growth and enabling laboratories and research centers to adopt advanced solutions. |
| Expansion of biobanking and personalized medicine | The rise of biobanking and personalized therapies increases demand for specialized collection kits that preserve genetic, molecular, and clinical samples for long-term research and patient-specific diagnostics. |
| Pitfalls & Challenges | Impact |
| High cost of advanced kits and complexities associated with specimen collection | Expensive collection kits restrict adoption in smaller laboratories and emerging markets, limiting accessibility and slowing overall market growth despite increasing global demand for high-quality specimens. |
| Opportunities: | Impact |
| Rising adoption of at-home and remote sample collection kits | Home-based and remote collection solutions expand accessibility, convenience, and patient engagement, driving future market growth and increasing penetration of decentralized diagnostic and telehealth services. |
| Integration of automation and digital tracking solutions | Automation and digital monitoring of samples improve traceability, reduce human error, and are expected to enhance laboratory efficiency, compliance, and global adoption in the coming years. |
| Market Leaders (2024) | |
| Market Leaders |
18.3% market share |
| Top Players |
Collective market share in 2024 is 57.1% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | India, China, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Biological Sample Collection Kits Market Trends
The increasing number of laboratory tests is a significant driver for the kits market. Rising prevalence of infectious diseases, chronic conditions, and genetic disorders has substantially increased the volume of diagnostic and monitoring tests performed in hospitals, clinics, and research laboratories.
- As healthcare systems expand and patient awareness grows, there is greater reliance on laboratory-based diagnostics to ensure early and accurate disease detection, which directly drives the demand for high-quality specimen collection kits. These kits including swabs, blood collection systems, and urine collection systems, as well as viral transport media, play a critical role in maintaining sample integrity, reducing contamination risks, and enabling reliable test results.
- Technological advancements further strengthen this driver by introducing automated, user-friendly, and high-throughput sample collection systems. Innovations such as flocked swabs, self-collection saliva kits, and temperature-stable transport media enhance convenience, accuracy, and turnaround times, allowing laboratories to handle increasing test volumes efficiently.
- Integration with digital tracking solutions, barcoding, and laboratory information systems (LIS) ensures precise sample traceability, improving workflow management and regulatory compliance.
- From a microeconomic perspective, individual laboratories and diagnostic service providers benefit from adopting advanced collection kits, as they reduce sample errors, improve patient satisfaction, and enable faster, high-quality diagnostic workflows.
- On a macroeconomic level, governments and healthcare authorities investing in national screening programs, biobanking, and public health surveillance contribute to consistent demand growth for collection kits. Additionally, economic growth and rising healthcare expenditure in emerging regions expand access to laboratory testing, further amplifying market opportunities.
- Overall, the convergence of rising laboratory test volumes, technological innovations in sample collection, and supportive economic conditions positions the market for robust growth in both developed and developing regions over the forecast period.
Biological Sample Collection Kits Market Analysis
Learn more about the key segments shaping this market
Based on the device type, the biological sample collection kits market is segmented into swabs, blood collection kits, urine collection kits, viral transport media, and other products. The blood collection kits segment has asserted its dominance in the market by securing a significant market share of 29.7% in 2024, driven by growing demand for routine diagnostics, chronic disease monitoring, and biomarker testing drives widespread adoption of reliable and standardized blood collection kits. The segment is expected to exceed USD 6.3 billion by 2034, growing at a CAGR of 8.4% during the forecast period.
On the other hand, the swabs segment is expected to grow with a CAGR of 7.2%. Rising demand for rapid infectious disease testing, microbiome studies, and non-invasive sample collection is driving steady growth in the swabs segment.
- The swabs segment is expected to grow due to its widespread use in diagnostics, particularly in infectious disease testing, including COVID-19, influenza, and sexually transmitted infections. Rising awareness of early and accurate disease detection has fueled demand for swabs across hospitals, diagnostic laboratories, and point-of-care settings.
- Technological innovations, such as flocked swabs and high-absorbency swabs, have enhanced sample collection efficiency and accuracy, thereby increasing adoption. Additionally, the shift toward home-based testing and self-collection kits, especially during the COVID-19 pandemic, has significantly contributed to swab sales.
- Regulatory approvals and standardization of swab types for specific tests have further increased market penetration. Moreover, expansion in emerging markets with improving healthcare infrastructure, coupled with government initiatives promoting preventive healthcare and routine screening programs, is propelling growth.
- The blood collection kits segment held a revenue of USD 2.7 billion in 2024, with projections indicating a steady expansion at 8.4% CAGR from 2025 to 2034. Blood collection kits are witnessing strong growth due to their critical role in clinical diagnostics, therapeutic monitoring, and disease screening. The rising prevalence of chronic diseases, such as diabetes, cardiovascular disorders, and cancer, has increased the demand for frequent blood tests, driving the adoption of blood collection kits.
- Innovations in blood collection technology, such as vacuum tubes, capillary collection systems, and integrated serum/plasma separation devices, have improved sample stability, safety, and ease of use. Additionally, the growing trend toward decentralized healthcare and home-based testing solutions has fueled the use of convenient and user-friendly blood collection kits.
- Regulatory support and standardization in phlebotomy and blood handling procedures also ensure reliable sample quality, further boosting adoption. The expanding clinical research and biotechnology sectors, which require consistent and high-quality blood samples for trials and diagnostics, represent a significant driver for this segment.
- The urine collection kits segment held a revenue of USD 2.4 billion in 2024, with projections indicating a steady expansion at 8.1% CAGR from 2025 to 2034. It is driven by rising demand for non-invasive diagnostic testing and increasing prevalence of chronic kidney disease, urinary tract infections, and diabetes.
- Growth in at-home healthcare and decentralized clinical trials has amplified the need for convenient, sterile, and reliable urine collection kits. Technological advancements, including preservative-containing kits, enhance sample integrity and accuracy.
- Additionally, expanding awareness of preventive healthcare, growing geriatric populations, and the adoption of point-of-care testing in hospitals and diagnostic laboratories further propel market growth. Regulatory approvals and increasing investments by key manufacturers also support segment expansion.
Based on application, the biological sample collection kits market is classified into diagnostics and research. The diagnostics segment dominated the market with a revenue share of 78.9% in 2024 and is expected to reach USD 15.8 billion within the forecast period.
- The diagnostics segment dominates the market, driven primarily by the rising prevalence of chronic and infectious diseases globally. Increasing incidence of conditions such as cancer, cardiovascular disorders, diabetes, and infectious outbreaks like COVID-19 has led to heightened demand for accurate and timely diagnostic testing.
- Biological sample collection kits play a crucial role in enabling early detection, which improves patient outcomes and reduces healthcare costs, thereby stimulating market growth.
- Technological advancements in sample collection, including easy-to-use, minimally invasive, and automated collection systems, have further fueled adoption in diagnostic laboratories, hospitals, and clinics.
- The integration of molecular diagnostics, immunoassays, and high-throughput screening techniques requires reliable and standardized collection kits to ensure sample integrity, enhancing their necessity in diagnostics.
- Additionally, increasing healthcare awareness among patients and growing government initiatives promoting early disease detection have expanded the market penetration of diagnostics-based kits.
- The research segment held a revenue of USD 1.9 billion in 2024, with projections indicating a steady expansion at 7.1% CAGR from 2025 to 2034. It is primarily driven by the exponential growth in biomedical and life sciences research.
- Rising investment in academic institutions, pharmaceutical companies, and biotechnology firms has fueled demand for reliable sample collection kits that maintain the integrity of biological specimens for preclinical studies, drug discovery, and translational research.
- The increasing focus on genomics, proteomics, and metabolomics research has created a substantial need for standardized and reproducible sample collection methods. Advanced research workflows require kits that can preserve RNA, DNA, proteins, and other biomolecules under controlled conditions, making high-quality collection kits indispensable for accurate analysis.
- The adoption of biobanking practices has also contributed significantly, as large-scale storage of clinical and experimental samples demands robust, contamination-free collection solutions.
Learn more about the key segments shaping this market
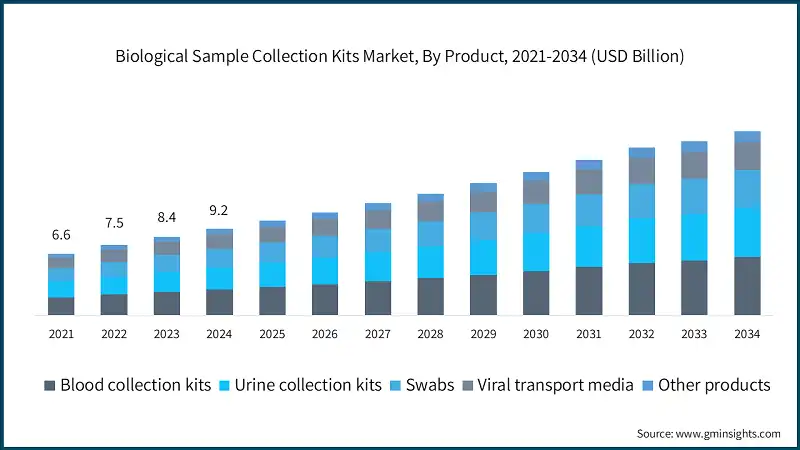
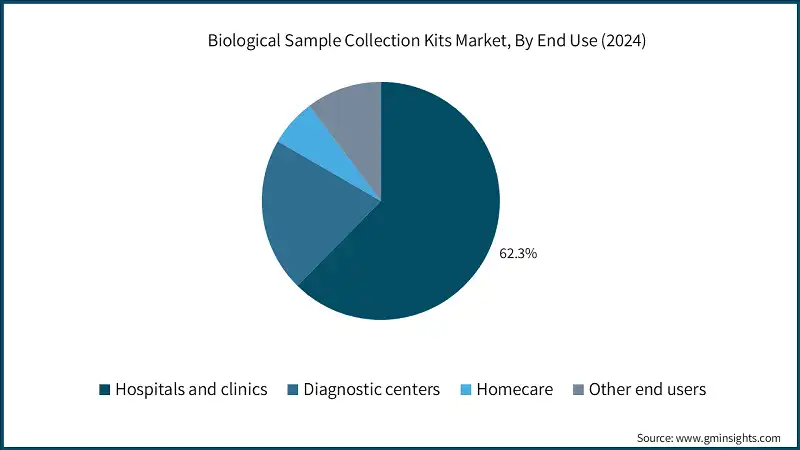
Based on end use, the biological sample collection kits market is classified into hospitals and clinics, diagnostic centers, homecare, and other end users. The hospitals and clinics segment dominated the market with a revenue share of 62.3% in 2024 and is expected to reach USD 12.2 billion within the forecast period.
- The two largest segments, namely hospitals and clinics, and diagnostic centers, account for over 83.3% of the total market value. These segments dominate the market due to the high volume of patient visits and diagnostic procedures performed in these settings. Hospitals are primary points of care for patients requiring routine check-ups, specialized testing, and emergency care, which drives continuous demand for reliable sample collection kits.
- The adoption of advanced diagnostic technologies and automated sample handling systems further fuels kit usage, ensuring accuracy, safety, and efficiency in clinical workflows.
- Moreover, hospitals and clinics increasingly focus on infection control and biohazard management, which necessitates high-quality kits designed to minimize contamination risks and improve sample integrity.
- Rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and infectious diseases increase the frequency of tests, contributing to sustained market growth.
- The diagnostic centers segment held a revenue of USD 1.9 billion in 2024, with projections indicating a steady expansion at an 8% CAGR from 2025 to 2034. Diagnostic centers are key drivers of the biological sample collection kits market due to their specialization in laboratory testing and high patient throughput. These centers perform a wide range of diagnostic tests, including pathology, molecular testing, and genetic analysis, which require precise and standardized sample collection to ensure reliable results.
- The increasing awareness of preventive healthcare and early disease detection has led to a surge in patients seeking diagnostic services, thereby boosting demand for collection kits. Diagnostic centers also adopt modern laboratory automation systems, which require compatible collection kits that maintain sample quality during processing and transportation.
- Additionally, the rise of specialized testing, including cancer biomarker analysis, infectious disease screening, and personalized medicine, emphasizes the need for accurate sample collection kits.
Looking for region specific data?
North America Biological Sample Collection Kits Market
North America dominated the market with the highest market share of 32.4% in 2024.
- North America dominated the market in 2024, largely driven by its advanced healthcare infrastructure, high adoption of cutting-edge diagnostic technologies, and strong presence of key market players. The region benefits from a well-established network of hospitals, diagnostic laboratories, and research centers that rely on accurate and safe sample collection for patient care, clinical trials, and research activities.
- Increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, cancer, and infectious diseases fuels the need for frequent diagnostic testing, thereby driving kit demand.
- Regulatory support from agencies like the FDA ensures high-quality and standardized kits, increasing healthcare provider confidence and adoption. Technological advancements, including automated sample handling systems, self-collection kits, and integrated digital health solutions, facilitate improved workflow efficiency and patient safety.
- Moreover, significant investments in R&D, personalized medicine, and genomics research create continuous demand for innovative biological sample collection solutions.
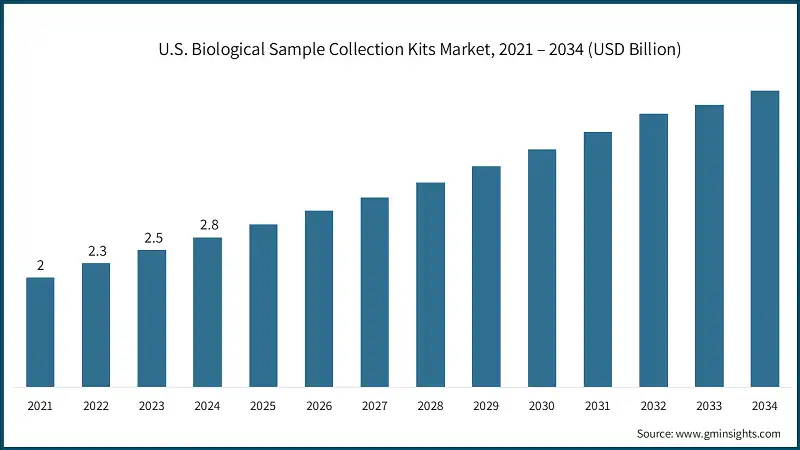
The U.S. biological sample collection kits market was valued at USD 2 billion and USD 2.3 billion in 2021 and 2022, respectively. In 2024, the market size grew to USD 2.8 billion from USD 2.5 billion in 2023.
- The U.S., the largest market in North America, benefits from an advanced healthcare ecosystem, strong regulatory support, and high awareness of preventive diagnostics.
- Hospitals, clinics, and diagnostic centers in the country rely heavily on biological sample collection kits due to high patient volumes and the national emphasis on early disease detection and chronic disease management.
- Growing incidences of cancer, diabetes, cardiovascular diseases, and infectious conditions drive the need for frequent diagnostic testing, directly increasing kit consumption. The U.S. also leads in adopting digital health solutions, telemedicine, and at-home self-collection kits, enabling convenient and remote diagnostics.
- The strong presence of major global and domestic kit manufacturers further supports continuous innovation, product availability, and efficient nationwide distribution.
Europe Biological Sample Collection Kits Market
Europe market accounted for USD 2.5 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- The European biological sample collection kits market is experiencing robust growth due to the region’s advanced healthcare infrastructure, increasing diagnostic testing, and high adoption of innovative medical technologies. Europe has a well-established network of hospitals, diagnostic centers, and research institutions that drive consistent demand for high-quality sample collection kits.
- Rising prevalence of chronic diseases, such as cardiovascular disorders, diabetes, and cancer, is fueling the need for frequent diagnostic testing, while government initiatives and public health programs aimed at improving disease detection and prevention further boost market demand.
- Technological advancements, including automated sample collection systems and novel self-collection kits, enhance the accuracy and efficiency of diagnostic workflows. The region’s focus on personalized medicine, genomics, and molecular diagnostics also drives adoption of kits suitable for specialized applications.
- Additionally, stringent regulations on biosafety, sample handling, and quality control encourage the use of certified and standardized kits. Increasing collaborations between healthcare providers, research institutions, and kit manufacturers support innovation and supply chain optimization.
Germany dominates the European biological sample collection kits market, showcasing strong growth potential.
- Germany, as Europe’s largest healthcare market, is a key growth driver for biological sample collection kits due to its advanced healthcare system, high patient awareness, and strong regulatory framework. Hospitals, diagnostic laboratories, and research centers in Germany actively adopt innovative sample collection solutions to support routine testing, preventive diagnostics, and clinical research activities.
- The rising prevalence of chronic diseases, such as diabetes, cardiovascular conditions, and cancer, which increases the demand for frequent and accurate diagnostic testing.
- Germany also places strong emphasis on quality, biosafety, and compliance with EU regulations, promoting the use of standardized, reliable collection kits. The adoption of digital health platforms and telemedicine services has accelerated home-based sample collection, making diagnostics more accessible and patient-friendly.
- Furthermore, Germany is a hub for clinical trials and biotechnology research, necessitating high-quality kits for specimen collection, transport, and storage.
Asia Pacific Biological Sample Collection Kits Market
The Asia Pacific market is anticipated to grow at the highest CAGR of 9.2% during the analysis timeframe.
- The Asia Pacific market is witnessing rapid growth, driven by a combination of expanding healthcare infrastructure, increasing prevalence of chronic and infectious diseases, and growing awareness of early diagnostics.
- Rapid urbanization and improving access to healthcare services in countries such as India, Japan, South Korea, and Southeast Asia are significantly boosting demand for biological sample collection kits across hospitals, diagnostic centers, and homecare settings.
- The region is also experiencing substantial government investments in healthcare modernization, digital health initiatives, and disease surveillance programs, which increase the adoption of standardized and high-quality collection kits. Moreover, the growing prevalence of diabetes, cardiovascular diseases, and infectious diseases such as tuberculosis and hepatitis drive frequent diagnostic testing, reinforcing kit demand.
- Expansion of private healthcare facilities and diagnostic laboratories, coupled with rising disposable incomes, has facilitated better access to advanced diagnostic solutions.
China biological sample collection kits market is estimated to grow with a significant CAGR, in the Asia Pacific market.
- China is expanding rapidly due to significant investments in healthcare infrastructure, diagnostic laboratories, and research facilities. The country has witnessed a surge in government initiatives aimed at improving early disease detection, infectious disease monitoring, and chronic disease management, which drive consistent demand for reliable sample collection kits.
- Rising prevalence of lifestyle-related chronic conditions, such as diabetes, cardiovascular disorders, and cancer, increases the frequency of diagnostic testing across hospitals, clinics, and specialized diagnostic centers.
- The COVID-19 pandemic further highlighted the importance of accurate and large-scale sample collection, prompting widespread adoption of automated and high-throughput collection systems.
- China’s rapidly growing population and urbanization have expanded healthcare accessibility, while increasing disposable incomes have improved affordability for advanced diagnostic services.
- The development of home healthcare and telemedicine solutions has accelerated demand for at-home sample collection kits, particularly in tier-2 and tier-3 cities.
Latin American Biological Sample Collection Kits Market
Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period.
- Brazil leads the Latin America market due to a combination of robust healthcare infrastructure, rising awareness of preventive diagnostics, and increasing investment in clinical laboratories. The country has a well-established network of hospitals, clinics, and diagnostic centers that conduct a high volume of routine and specialized tests, fueling demand for reliable collection kits.
- The growing prevalence of chronic diseases, such as diabetes, cardiovascular disorders, and infectious diseases like Zika and dengue, drives the need for more frequent diagnostic testing.
- Government initiatives, including public health programs to improve access to diagnostic services and expand laboratory capabilities, further support market growth.
- Technological advancements, such as automated sample processing, self-collection kits, and point-of-care testing solutions are increasingly being adopted by healthcare providers to improve efficiency and reduce errors.
Middle East and Africa Biological Sample Collection Kits Market
Saudi Arabia market to experience substantial growth in the Middle East and Africa market in 2024.
- Saudi Arabia’s market is driven by the government’s significant investment in healthcare modernization under the Vision 2030 initiative, which emphasizes the expansion of hospitals, diagnostic centers, and research facilities.
- The country has witnessed rapid growth in healthcare infrastructure, including specialized laboratories and advanced clinical testing facilities, boosting demand for high-quality collection kits.
- Rising prevalence of lifestyle-related chronic diseases, such as diabetes, obesity, and cardiovascular disorders, necessitates frequent laboratory testing, while infectious disease monitoring, including COVID-19 and MERS-CoV, has heightened awareness about accurate and safe sample collection.
- The increasing adoption of homecare testing and telemedicine solutions is also contributing to market expansion, enabling patients to collect samples conveniently and safely. Saudi Arabia’s focus on research and development, particularly in biotechnology, pharmaceuticals, and personalized medicine, also drives demand for kits in clinical trials and laboratory research.
Biological Sample Collection Kits Market Share
The global biological sample collection kits market is highly competitive, with leading medical device and diagnostic companies focusing on product innovation, advanced technologies, and strategic collaborations to strengthen their market positions. Rising prevalence of chronic and infectious diseases, increasing patient awareness, and growing adoption of preventive diagnostic testing are driving companies to leverage R&D investments, digital health integration, and connected laboratory systems to enhance sample accuracy and improve patient outcomes. The global shift toward home-based testing, personalized medicine, and value-driven healthcare is also encouraging players to develop cost-effective, user-friendly, and patient-centric solutions while expanding their presence in emerging markets.
Key players include Thermo Fisher Scientific, Roche, QIAGEN, Abbott, and Becton, Dickinson and Company (BD), collectively accounting for 57.1% of the global market. These companies maintain leadership through extensive product portfolios, strong global distribution networks, and continuous advancements in blood, urine, saliva, and molecular sample collection kits. Their dominance is reinforced by strategic partnerships with hospitals, diagnostic centers, homecare providers, and research laboratories to enhance accessibility, reliability, and adoption.
Smaller and niche players are also gaining traction by focusing on portable, disease-specific, and self-collection kits. Competitive differentiation is increasingly defined by the ability to deliver technologically advanced, safe, and cost-efficient sample collection solutions tailored to diverse healthcare settings. As the market evolves, competition is expected to intensify, with both established leaders and emerging firms pursuing innovation, digital integration, and strategic alliances to capture greater market share.
Biological Sample Collection Kits Market Companies
Few of the prominent players operating in the biological sample collection kits industry include:
- Abbott
- Altona Diagnostics
- Becton, Dickinson and Company (BD)
- CTK Biotech
- Hardy Diagnostics
- HiMedia Laboratories
- Labcorp
- Lucence Health
- Miraclean Technology
- Puritan Medical
- QIAGEN
- Roche
- Seegene
- Thermo Fisher Scientific
- VIRCELL MEDICAL
- Thermo Fisher Scientific
Thermo Fisher Scientific leads the biological sample collection kits market with a share of 18.3% in 2024. Thermo Fisher Scientific offers a comprehensive range of high-quality biological sample collection kits, emphasizing precision, reliability, and biosafety. Its solutions support hospitals, laboratories, and research facilities with automated, standardized, and easy-to-use kits for blood, saliva, and molecular testing. Strong global distribution and innovation in sample integrity and preservation differentiate the brand.
Roche provides advanced, clinically validated biological sample collection kits designed for accurate diagnostics and research applications. Its kits are integrated with cutting-edge molecular and immunoassay technologies, ensuring sample stability, reproducibility, and safety. Strategic partnerships with hospitals, diagnostic centers, and laboratories, along with robust quality standards, enhance accessibility and adoption globally.
Biological Sample Collection Kits Industry News:
- In October 2022, CVS Health partnered with Ixlayer to provide a home test kit capable of detecting Lyme disease, thyroid function, sexually transmitted infections, and Vitamin D levels. The collaboration expanded CVS’s at-home testing offerings, enabling convenient, accurate, and comprehensive diagnostic solutions for consumers seeking preventive healthcare from home.
- In June 2022, altona Diagnostics GmbH introduced the RealStar Zoonotic Orthopoxvirus PCR Kit 1.0, a real-time RT-PCR reagent system. This launch strengthened the company’s competitive position, expanded its molecular diagnostics portfolio, and enhanced its reputation for providing reliable, advanced solutions for detecting zoonotic orthopoxviruses across clinical and research settings.
- In May 2022, Labcorp announced the development of a home collection diabetes kit that measures hemoglobin A1c (HbA1c) from a small blood sample. This launch enabled convenient monitoring of blood sugar levels, improved patient engagement, and strengthened Labcorp’s position in the growing home-based diagnostic testing segment for diabetes management.
- In July 2021, Abbott launched the Panbio COVID-19 Antigen Self-Test in India, expanding its rapid diagnostics portfolio to address growing testing demands. The product contributed to revenue growth, increased brand recognition, and broadened the customer base by offering accessible, easy-to-use self-testing solutions for COVID-19 detection across urban and rural regions.
The biological sample collection kits market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD s from 2021 – 2034 for the following segments:
Market, By Product
- Blood collection kits
- Urine collection kits
- Swabs
- Nasopharyngeal (NP) swabs
- Oropharyngeal (OP) swabs
- Nasal swabs
- Viral transport media
- Other products
Market, By Application
- Diagnostics
- Research
Market, By End Use
- Hospitals and clinics
- Diagnostic centers
- Homecare
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which region leads the biological sample collection kits market?
North America led the market with a 32.4% share in 2024, driven by advanced healthcare infrastructure, high diagnostic testing rates, and significant biobanking activities.
What are the upcoming trends in the biological sample collection kits market?
Key trends include the adoption of automated and user-friendly sample collection systems, innovations in temperature-stable transport media, and the growing use of self-collection kits for convenience and efficiency.
Who are the key players in the biological sample collection kits market?
Key players include Abbott, Altona Diagnostics, Becton, Dickinson and Company (BD), CTK Biotech, Hardy Diagnostics, HiMedia Laboratories, and Labcorp.
What was the valuation of the diagnostics segment in 2024?
The diagnostics segment held a revenue share of 78.9% in 2024, generating USD 7.26 billion, and is expected to reach USD 15.8 billion by 2034.
Which end-use segment leads the biological sample collection kits market?
The hospitals and clinics segment dominated the market with a 62.3% revenue share in 2024, and is projected to reach USD 12.2 billion by 2034.
What is the projected size of the biological sample collection kits market in 2025?
The market is expected to reach USD 10.1 billion in 2025.
How much revenue did the blood collection kits segment generate?
The blood collection kits segment accounting for 29.7% of the market share, and is projected to exceed USD 6.3 billion by 2034 with a CAGR of 8.4% during the forecast period.
What is the market size of the biological sample collection kits market in 2024?
The market size was USD 9.2 billion in 2024, with a CAGR of 7.7% expected through 2034, driven by increasing demand for diagnostic testing, biobanking activities, and advancements in personalized medicine biomarkers.
What is the projected value of the biological sample collection kits market by 2034?
The market is expected to reach USD 19.7 billion by 2034, fueled by technological advancements, rising prevalence of chronic and infectious diseases, and growing reliance on laboratory-based diagnostics.
Biological Sample Collection Kits Market Scope
Related Reports


