Too Rare to Cure? How AI is Proving That Wrong
Published Date: September 25, 2025
Rare diseases, also known as orphan diseases, affect a small portion of the population, typically fewer than 1 in 2,000 individuals. While each disease is uncommon, collectively they impact over 300 million people worldwide, cutting across all regions and demographics. Despite this significant global burden, over 90% of rare diseases still lack approved treatments, leaving millions without effective therapeutic options.
Historically, drug development for rare diseases has been hindered by challenges such as small patient populations, complex disease biology, and fragmented data. However, advancements in artificial intelligence (AI) are now revolutionizing this field, driving faster orphan drug discovery, and paving the way for innovative solutions in rare disease drug development.
On the other hand, rare disease drug development faces significant challenges due to the unique nature of these conditions. Small, dispersed patient populations make clinical trials difficult to conduct, while genetic variability complicates the identification of therapeutic targets and the development of personalized treatments. High research and development costs, combined with uncertain market returns, often deter pharmaceutical companies from pursuing orphan drug programs. Additionally, the lack of comprehensive, high-quality data, particularly in low- and middle-income regions, hinders the effectiveness of traditional research approaches. These factors have historically contributed to limited progress in addressing the unmet medical needs of rare disease patients.

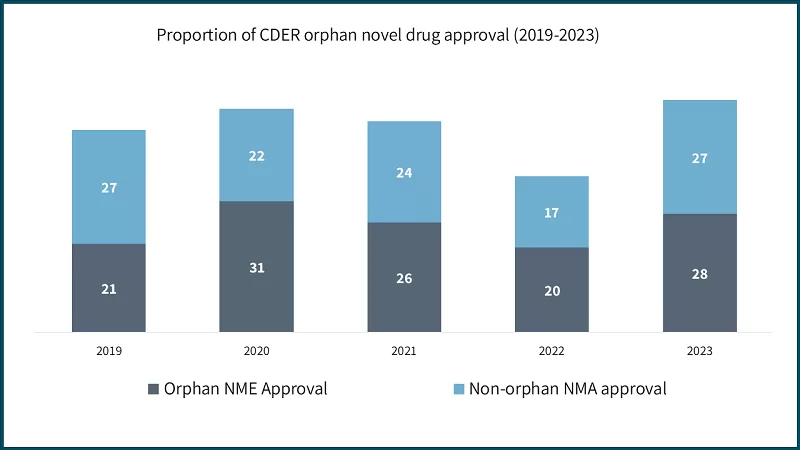
Recognizing these challenges, the FDA’s Center for Drug Evaluation and Research (CDER) launched the Accelerating Rare disease Cures (ARC) Program to promote innovative scientific approaches and regulatory clarity. Alongside this, CDER and the Center for Biologics Evaluation and Research (CBER) are establishing the Rare Disease Innovation Hub to support cross-center collaboration and regulatory science, especially for diseases with variable natural histories.
These efforts align with AI’s capabilities in modelling, biomarker discovery, and real-world evidence generation. Additionally, expedited review pathways, fast track, breakthrough therapy, priority review, and accelerated approval, are helping bring promising therapies to patients faster, with 88% of CDER approvals from 2015–2023 using at least one such program.
AI: A game-changer in orphan drug discovery
AI is fundamentally changing how researchers approach rare disease drug development. By addressing the traditional barriers that have long delayed progress, AI technologies are creating new possibilities at every stage of drug discovery. For conditions that have historically received limited attention due to their complexity and small patient populations, AI offers renewed hope by making the development process more efficient and effective.
1. Accelerated target identification
AI-powered algorithms are transforming the early stages of drug discovery by analysing vast and diverse datasets, including genomic, proteomic, and clinical data. These advanced tools can uncover hidden patterns and disease mechanisms that traditional methods often overlook, particularly in genetically complex conditions.
By identifying novel drug targets with greater speed and precision, AI significantly reduces the time required for the discovery phase. This acceleration is critical in rare disease research, where understanding the underlying biology is often the most challenging and time-consuming step. Furthermore, AI enables researchers to prioritize targets based on their potential therapeutic impact, ensuring resources are allocated efficiently.
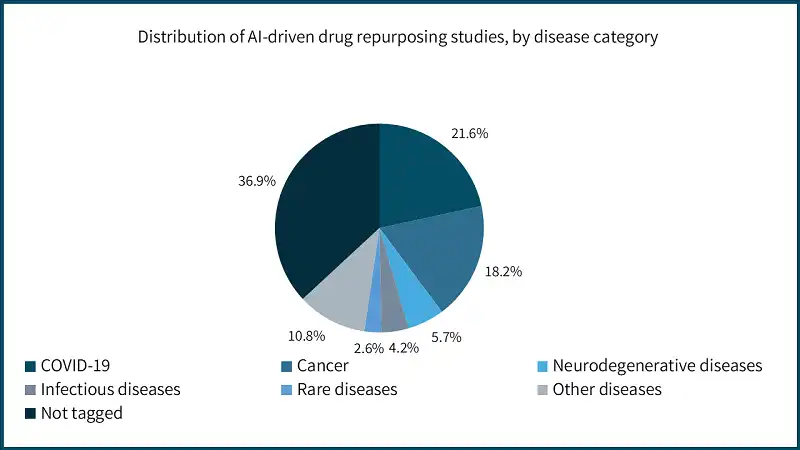
2. Drug repurposing opportunities
AI is proving to be a powerful tool for identifying existing drugs that can be repurposed to treat rare diseases. By mining extensive databases of approved compounds and comparing them to disease-specific molecular signatures, AI can suggest candidates that may bypass the need for early-stage development. This approach not only saves significant time and costs but also leverages the existing safety and efficacy data of approved drugs, increasing the likelihood of regulatory approval.
Drug repurposing through AI is particularly valuable for rare diseases, where the small patient populations often make traditional drug development economically unviable. This strategy is opening new avenues for providing treatments to underserved communities.
3. Predictive modelling for clinical trials
AI-driven predictive modelling is reshaping the design and execution of clinical trials, especially for rare diseases where patient recruitment is inherently limited. These models simulate disease progression and predict treatment responses, enabling researchers to design smarter, more efficient trials. AI also supports adaptive trial designs, allowing for real-time adjustments based on emerging data. This flexibility is crucial in rare disease research, where trial endpoints may be unclear, and patient heterogeneity can complicate outcomes. By improving trial efficiency and success rates, AI is helping to bring life-saving treatments to market faster and more cost-effectively.
4. Literature mining with NLP
Natural Language Processing (NLP), a subset of AI, is revolutionizing the way researchers access and utilize scientific literature. NLP tools can scan and analyse thousands of scientific papers, patents, and clinical trial records to extract relevant insights in a fraction of the time it would take manually. This capability accelerates hypothesis generation, identifies gaps in current knowledge, and ensures researchers stay updated on the latest findings. Additionally, NLP aids in mapping disease ontologies and therapeutic pathways, providing a comprehensive understanding of rare diseases. By synthesizing vast amounts of information, NLP empowers researchers to make data-driven decisions with greater confidence.
5. Biomarker discovery and patient stratification
AI is playing a pivotal role in identifying biomarkers that are essential for disease diagnosis, treatment response, and patient stratification. Biomarkers enable personalized medicine approaches, which are particularly critical in rare diseases where genetic and phenotypic heterogeneity can significantly impact drug efficacy. AI algorithms analyse complex datasets to uncover these biomarkers, facilitating the development of targeted therapies. By stratifying patients based on specific genetic or phenotypic markers, researchers can design more effective clinical trials and improve therapeutic outcomes. This personalized approach not only enhances the likelihood of trial success but also ensures that treatments are tailored to the unique needs of each patient group.
In conclusion, AI is proving to be a transformative force in rare disease drug development, addressing the unique challenges of this field with innovative solutions. By accelerating target identification, enabling drug repurposing, optimizing clinical trials, mining scientific literature, and advancing biomarker discovery, AI is paving the way for a new era of precision medicine. As these technologies continue to evolve, they hold the potential to bring hope and effective treatments to millions of patients affected by rare diseases worldwide.
How technology advancements are transforming rare disease drug development
Rare disease drug development has historically faced significant challenges, but advancements in technology are now paving the way for more efficient and effective solutions. Artificial intelligence (AI) is at the forefront of this transformation, offering tools that streamline processes and enhance precision. One of the most impactful innovations is the adoption of cloud-based platforms, which enable seamless and secure data sharing across research institutions, pharmaceutical companies, and healthcare providers.
This connectivity addresses the issue of fragmented datasets, allowing researchers to pool resources and insights to better understand rare conditions. By fostering collaboration on a global scale, these platforms are accelerating the pace of discovery and innovation in rare disease research.
Federated learning: unlocking decentralized data: Another groundbreaking development is the implementation of federated learning models. These models allow AI algorithms to analyse decentralized data sources without compromising patient privacy, a critical consideration in rare disease research where data is often limited and sensitive. Federated learning ensures that researchers can access diverse datasets while maintaining compliance with stringent data protection regulations.
This approach not only enhances the quality of insights derived from the data but also encourages participation from institutions that may have previously hesitated due to privacy concerns. By unlocking the potential of underutilized data, federated learning is helping to overcome one of the most significant barriers in rare disease drug development.
Digital twins: enabling personalized drug testing: Digital twin technology is also revolutionizing the field by enabling personalized medicine approaches. Digital twins are virtual simulations of individual patient biology, created using data from genetic, phenotypic, and clinical sources. These simulations allow researchers to test drug responses and predict outcomes in a virtual environment before initiating clinical trials. This capability is particularly valuable in rare diseases, where patient populations are small and heterogeneous. By providing a deeper understanding of how a drug might interact with specific patient profiles, digital twins reduce the risk of trial failures and improve the likelihood of successful outcomes. This technology is not only enhancing the precision of drug development but also reducing costs and timelines.
AI-powered lab automation: accelerating discovery: Additionally, AI-powered lab automation is streamlining critical processes such as compound screening, assay development, and validation. Automated systems can handle large volumes of data and repetitive tasks with high accuracy, significantly reducing the potential for human error. This increased efficiency allows researchers to focus on more complex aspects of drug development, such as analysing results and refining hypotheses. Moreover, automation accelerates the overall timeline of drug discovery, which is crucial for rare disease patients who often face long waits for new treatments. By integrating automation into the workflow, the industry is making strides toward faster and more reliable drug development processes.
These technological advancements are collectively transforming the landscape of rare disease drug development. By addressing long-standing challenges such as data fragmentation, patient privacy, and resource limitations, they are enabling researchers to make meaningful progress in understanding and treating rare conditions. As these innovations continue to evolve, they hold the promise of not only improving the efficiency of drug development but also bringing hope to millions of patients worldwide who are waiting for life-changing therapies.
What’s next: the future of AI in rare disease therapeutics
1. Multi-omics integration: AI is increasingly being used to integrate data from genomics, transcriptomics, proteomics, and metabolomics. This holistic view of disease biology enables deeper insights and more targeted interventions.
2. Real-world evidence and patient registries: AI tools are mining real-world data from electronic health records, patient registries, and wearable devices to inform drug development. This helps identify unmet needs, monitor long-term outcomes, and refine treatment strategies.
3. AI-driven gene therapy design: AI is playing a growing role in designing gene therapies for monogenic rare diseases. By predicting off-target effects and optimizing delivery vectors, AI is helping make gene editing safer and more effective.
4. Collaborative ecosystems: Pharma companies, AI startups, academic institutions, and patient advocacy groups are forming collaborative ecosystems to accelerate innovation. Regulatory bodies are also adapting to evaluate AI-based drug development tools, paving the way for broader adoption.
Challenges hindering broader adoption
Despite its transformative potential, the adoption of artificial intelligence in orphan drug discovery faces several critical challenges that must be addressed to unlock its full impact.
1. Data scarcity and quality
One of the most pressing challenges in applying AI to rare disease drug development is the lack of large, high-quality datasets. Rare diseases inherently affect small populations, making it difficult to gather statistically significant and diverse data. AI models require robust, well-annotated datasets to train effectively, and limited availability can lead to biased outputs, reduced accuracy, and poor generalizability. This data scarcity hampers the ability of AI to identify reliable biomarkers, predict drug responses, or stratify patients. Addressing this issue requires global data-sharing initiatives, standardized data formats, and incentives for institutions to contribute anonymized patient data.
2. Regulatory uncertainty
Global regulatory frameworks for AI in drug development are still evolving, and this lack of harmonization poses a significant barrier. Different regions have varying standards for validating AI algorithms, approving AI-assisted clinical trials, and ensuring transparency in decision-making. This regulatory ambiguity can slow down drug approvals, create compliance hurdles, and discourage investment in AI-driven innovation. To unlock the full potential of AI in rare disease R&D, regulators must establish clear, consistent guidelines that balance innovation with patient safety and ethical oversight.
3. Ethical and privacy concerns
Using patient data to train AI models raises critical ethical questions around consent, privacy, and data ownership. Rare disease data is often highly sensitive and identifiable due to the uniqueness of patient profiles. Without transparent governance and secure data handling protocols, there is a risk of data misuse, breaches, or exploitation. Institutions may hesitate to share data, fearing reputational or legal consequences. To mitigate these concerns, AI developers must implement privacy-preserving techniques (for instance, federated learning), ensure informed consent, and adhere to global data protection regulations such as GDPR or HIPAA.
4. Cost and infrastructure
Implementing AI solutions in rare disease drug development requires substantial investment in computational infrastructure, skilled personnel, and data management systems. Smaller biotech firms and academic labs may lack the resources to deploy advanced AI tools or maintain high-performance computing environments. This creates a disparity in innovation capacity, where only well-funded organizations can fully leverage AI. Strategic partnerships, cloud-based platforms, and open-source AI frameworks can help democratize access and reduce barriers for emerging players in the field.
Conclusion: A new era for rare disease treatment
Artificial intelligence (AI) represents more than just a technological advancement; it signifies a transformative shift in addressing some of the most complex challenges in medicine. In the realm of rare diseases, where every patient and discovery hold immense value, AI is accelerating orphan drug development by making the process more efficient, precise, and inclusive.
As AI technology advances, its application in rare disease research and development is unlocking unprecedented opportunities. These include predictive diagnostics, personalized treatment options, enhanced global collaborations, and streamlined regulatory processes. The future of rare disease drug development is now driven by the intelligent integration of data, algorithms, and human expertise, overcoming traditional limitations.





