Biological Safety Testing Market size to exceed $4.5 bn by 2025
Published Date: November 2019
Biological Safety Testing Market size is set to exceed USD 4.5 billion by 2025; according to a new research report by Global Market Insights Inc.
Rising need to maintain drug quality and safety will lead to increase in demand for biological safety testing. Presence of strict guidelines and policies for high-quality manufacturing of pharmaceutical products will lead to market expansion in the forthcoming years.
Controlled goods manufacturing and laboratory testing services drive the market revenue
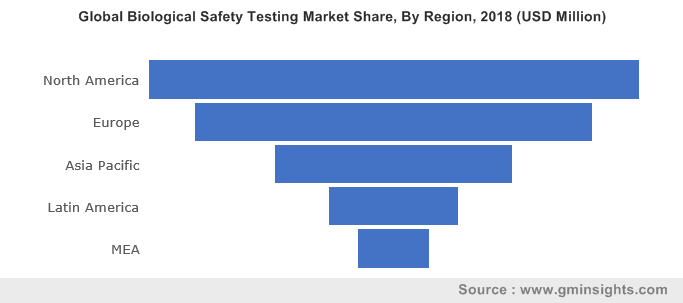
Get more details on this report - Request Free Sample PDF
Increasing emphasis on drug discovery and development of novel therapies to treat wide range of chronic diseases will offer attractive opportunities for biological safety testing market growth. Pharmaceutical products including parenteral preparations require stability testing to ensure drug longevity and pyrogen testing to assure pathogen-free biologics. Additionally, rising need for faster and high-quality drug development & discovery will further impel the adoption of biological safety testing.
Increasing number of pharmaceutical and biotechnology firms will offer lucrative growth potential to biological safety testing business. Several companies across developed countries such as the U.S. and Japan are investing in innovating new products and adopting contract manufacturing and research services. Also, numerous start-ups are providing research services for novel product development. Thus, with rising spending on pharmaceutical sector coupled with adoption of biological safety testing services, the market will witness significant growth in the upcoming years.
Browse key industry insights spread across 150 pages with 145 market data tables & 8 figures & charts from the report, Biological Safety Testing Market share, By Products and Services (Products {Kits and Reagents, Instruments}, Services), By Test (Endotoxin Tests, Sterility Tests, Adventitious Agent Detection Tests), By Application (Blood & Blood-based Products, Vaccines & Therapeutics, Stem Cells), By End-use (Pharmaceutical and Biotechnology Companies, Contract Development and Manufacturing Organization, Research and Academia), Industry Analysis Report, Regional Outlook, Application Potential, Competitive Market Share & Forecast, 2019 – 2025 in detail along with the table of contents:
https://www.gminsights.com/industry-analysis/biological-safety-testing-market
Advanced biological safety instruments will offer robust expansion opportunities
Rising need to store biological safety testing data will increase the demand for biological safety cabinets. Several organizations are focusing on development and launch of cloud-based biological safety testing to record, analyze, and monitor product safety information, easily and accurately. Hence, growing need to store bioanalytical information will spur the installation of biological safety testing instruments in the forthcoming years. However, high cost of biological safety instruments including biosafety cabinets may hinder the demand for biological safety testing market growth over the analysis period.
Growing demand for in-vitro assays for biological safety testing will boost the demand for endotoxin test
Endotoxin tests segment is projected to witness 12.2% CAGR over the forecast period. Endotoxin test is used to detect the presence and concentration of bacterial contamination in drugs and biological products. Key advantage associated with endotoxin test include enhanced product safety by controlling bacterial contamination during product development. Moreover, numerous safety testing service providers are offering in-vivo and in-vitro assays, that will positively impact the segmental growth over the estimation timeframe.
Growing blood-borne infections to generate lucrative demand for biological safety testing
Blood and blood-based products segment was valued at over USD 219.0 million in 2018. Increasing cases of bacterial contamination in blood products such as plasma and platelets escalates the need for biological safety testing. Blood and blood-based products are highly susceptible to contaminants and thus need to be pathogen-free before transfusion procedure. These products acutely monitored for safety and microbial contamination, thus driving the segmental growth.
Research and academic institutes stimulate demand for biological safety testing
Research and academia segment is projected to witness rapid growth over the forecast period. Rising preference of healthcare firms for development of biologics in research institutes will act as the major factor impacting the segmental growth. Research and academic institutes maintain strict regulatory standards and have technologically advanced equipment, that is suitable for biological safety testing. In addition, availability of proper biological safety testing protocol along with sophisticated infrastructure will impel the segmental growth over the projected period.
Rising market of drug development services in India will create demand for biological safety testing
India biological safety testing market is projected to witness over 12.5% CAGR during the analysis period. The Indian drugs manufacturing market is witnessing robust demand from established companies to manufacture biopharmaceutical products due to better affordability. Thus, with growing CDMO market, the need for biological safety testig products will accelerate in the future. In addition, increasing awareness regarding controlled goods manufacturing practices and product quality standards will highly impact the market growth.
Canada biological safety testing market accounted for over USD 80 million in revenue size in 2018. Increasing number of pharma and biotech companies coupled with rising inclination towards contract research services in the country will serve as the major factors augmenting the market growth. Moreover, rapid expansion of research laboratories and increasing funding for R&D in biologics will consistently contribute towards robust market growth.
Expansion of product offerings through inorganic strategic alliances encourage market players to enter in safety testing business
Alliance of industry players with established biosafety testing firms to offer biological safety testing services offers momentous growth potential to key market players. Some of the major market players involved in biological safety testing market include Eurofins Scientific, Merck KGaA, Lonza, Charles River, Sartorius AG, and SGS SA, among several others. In April 2015, Sartorius Stedim Biotech acquired contract biological testing service firm BioOutsource Ltd. The acquisition was aimed at extending business biological safety testing service across the U.S. and Asian countries.
Sumant Ugalmugle, Rupali Swain