Summary
Table of Content

Ventricular Assist Devices Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Ventricular Assist Devices Market Size
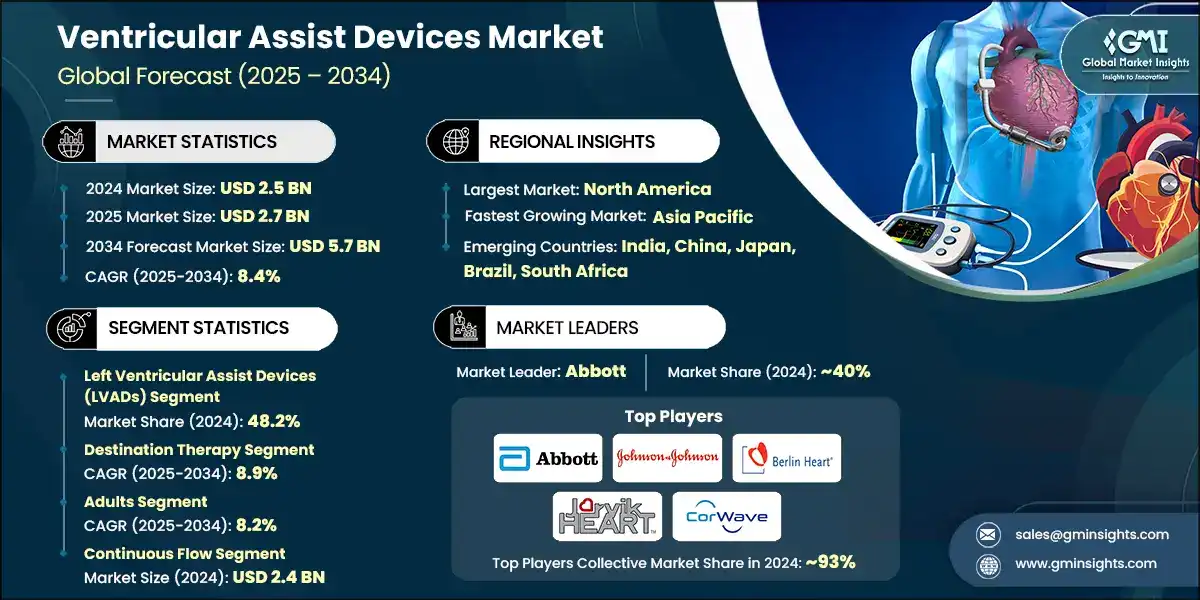
The global ventricular assist devices market was valued at USD 2.5 billion in 2024. The market is expected to grow from USD 2.7 billion in 2025 to USD 5.7 billion in 2034, at a CAGR of 8.4% during the forecast period, according to the latest report published by Global Market Insights Inc. The high market growth is attributed to the increase in the number of heart failures and cardiovascular diseases, rising awareness regarding heart failure treatment, and a shortage of heart donors, among other factors.
To get key market trends
Ventricular assist devices (VADs) provide therapeutic aid for patients with severe heart failure by mechanically augmenting cardiac output, either on a temporary or permanent basis. Abbott, Johnson & Johnson, Berlin Heart, and Jarvik Heart are major players in the VAD market, among others. These manufacturers produce a range of implantable VADs, percutaneous micro-axial pumps, and paracorporeal external systems, for both acute care facilities and long-term outpatient therapy.
The market has increased from USD 1.7 billion in 2021 and reached USD 2.2 billion in 2023, with a historic growth rate of 13.5%. This was mainly propelled by increased prevalence of advanced heart failure, device design-related technological advancements, and broadening applications in various healthcare settings.
According to the National Institutes of Health, as of 2021, heart failure had reached an estimated 55.5 million individuals globally, which is close to doubling from the 1990 number of 25.4 million cases. This increase primarily results from population aging, better survival rates for other cardiovascular diseases, and comorbidities such as hypertension, diabetes, and obesity.
Additionally, the burden of heart failure is increased by lifestyle factors and healthcare access disparities. For instance, in the U.S., adults over 65 years have a heart failure prevalence of 8.0%–9.1%, nearly four times higher than younger adults. In China, heart failure prevalence rose from 4.68 million in 1990 to 13.1 million in 2021, with an annual growth rate of 3.18%, outpacing global trends. These statistics underscore the urgent need for advanced therapeutic solutions like VADs, which offer life-saving support for patients with end-stage heart failure.
A ventricular assist device (VAD) is a mechanical pump that supports heart function and blood flow in individuals with weakened hearts. It helps circulate blood from the ventricles to the rest of the body, either temporarily or permanently, depending on the patient's condition.
Ventricular Assist Devices Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 2.5 Billion |
| Market Size in 2025 | USD 2.7 Billion |
| Forecast Period 2025 - 2034 CAGR | 8.4% |
| Market Size in 2034 | USD 5.7 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increase in the number of heart failures and cardiovascular diseases | Rising global prevalence of end-stage heart failure is fueling demand for mechanical circulatory support. |
| Technological advancements | Innovations in miniaturization, biocompatibility, and wireless systems are improving device performance and patient outcomes. |
| Rise in awareness regarding heart failure treatment | Greater public and clinical awareness is accelerating early diagnosis and adoption of advanced therapies like VADs. |
| Shortage of heart donors | Limited availability of donor hearts is increasing reliance on VADs as both bridge-to-transplant and destination therapy. |
| Pitfalls & Challenges | Impact |
| High cost of devices | The substantial expense of VAD implantation and maintenance limits accessibility, especially in low-resource settings. |
| Surgical risks and complications | Invasive procedures carry risks such as infection, bleeding, and device malfunction, impacting adoption and outcomes. |
| Opportunities: | Impact |
| Adoption in emerging markets | Expanding healthcare infrastructure and rising cardiac disease burden in developing regions present significant growth potential. |
| Growing demand for patient-friendly solutions | The shift toward less invasive, wearable, and home-monitorable devices is driving innovation and market expansion. |
| Market Leaders (2024) | |
| Market Leaders |
~40% market share |
| Top Players |
Collective market share in 2024 is Collective Market Share ~93% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Countries | India, China, Japan, Brazil, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Ventricular Assist Devices Market Trends
Technological advancements have significantly reshaped the market, enhancing device performance, safety, and patient outcomes.
- Innovations such as magnetic levitation (MagLev) technology, seen in Abbott’s HeartMate 3, have reduced mechanical wear and blood damage, improving long-term durability and reducing complications like thrombosis.
- Similarly, miniaturization of components has enabled more anatomically compatible devices, making implantation less invasive and expanding eligibility to a broader patient population.
- Moreover, the integration of wireless energy transfer, remote monitoring, and smart sensors is transforming VADs into connected therapeutic systems. These features allow clinicians to track device performance and patient vitals in real time, enabling proactive management and reducing hospital readmissions.
- Furthermore, biocompatible materials, adaptive flow algorithms, and fully implantable systems under development are pushing the boundaries of patient comfort and mobility.
- Thus, these advancements are not only improving clinical outcomes but also driving wider adoption across both developed and emerging healthcare markets.
Ventricular Assist Devices Market Analysis
Learn more about the key segments shaping this market
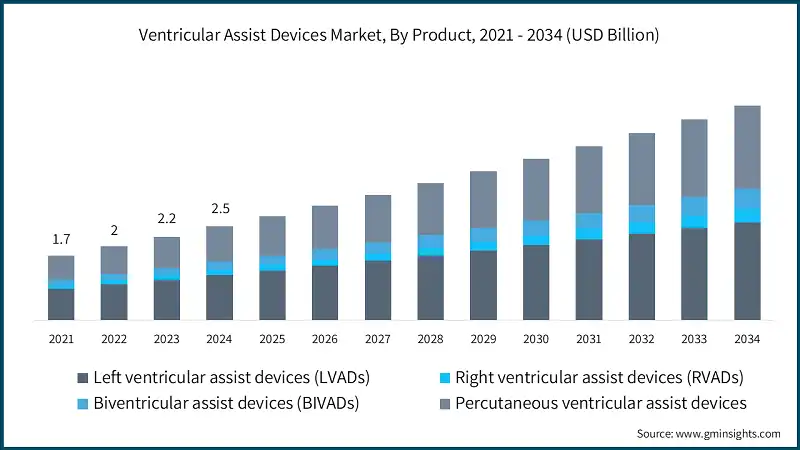
Based on the product, the ventricular assist devices market is segmented into left ventricular assist devices (LVADs), right ventricular assist devices (RVADs), biventricular assist devices (BIVADs), and percutaneous ventricular assist devices. The left ventricular assist devices (LVADs) segment accounted for 48.2% of the market in 2024 due to its increasing adoption in patients with end-stage heart failure, rising prevalence of cardiovascular diseases, and advancements in device technology improving patient survival and quality of life. The segment is expected to exceed USD 2.6 billion by 2034, growing at a CAGR of 7.8% during the forecast period.
On the other hand, the percutaneous ventricular assist devices (pVADs) segment held a market share of 37.4% in 2024, and its growth can be attributed to the increasing adoption of minimally invasive procedures, which allow for rapid deployment in catheterization labs without the need for open-heart surgery.
- This dominance of LVADs is driven by the widespread use of these devices in treating end-stage heart failure, particularly in bridge-to-transplant and destination therapy applications. LVADs have become the preferred option due to their proven clinical outcomes, technological advancements like continuous-flow systems, and expanded reimbursement coverage in key markets.
- The right ventricular assist devices (RVADs) segment is expected to grow with a 9.4% CAGR during the analysis period. The segment is primarily driven by the increasing incidence of right-sided heart failure, advancements in miniaturized and biocompatible device designs, and the growing use of RVADs in bridge-to-transplant and recovery therapies, especially in complex cardiac cases requiring targeted ventricular support.
- The market for the biventricular assist devices (BIVADs) segment is expected to expand rapidly, driven primarily by the increasing number of patients with biventricular heart failure, advancements in dual-pump configurations, and the growing use of BiVADs as a bridge to transplant or recovery in complex cardiac cases where single-ventricle support is insufficient.
Based on application, the ventricular assist devices market is segmented into destination therapy, bridge-to-candidacy (BTC) therapy, bridge-to-transplant (BTT) therapy, bridge-to-recovery (BTR) therapy, and other applications. The destination therapy segment dominated the market in 2024 and is growing with a CAGR of 8.9% during the forecast period.
- This growth is primarily driven by the increasing adoption of long-term mechanical circulatory support for patients ineligible for heart transplants, improved device durability, and clinical evidence showing survival rates comparable to transplant outcomes, especially among younger cohorts.
- The second largest segment, bridge-to-transplant (BTT) therapy, held a market share of 25.9% in 2024. This segment continues to be supported by decades of clinical success, established surgical protocols, and favorable reimbursement frameworks, making it a cornerstone of mechanical circulatory support for patients awaiting heart transplantation.
- The bridge-to-candidacy (BTC) therapy segment is expected to grow with a 7.8% CAGR during the analysis period. This growth is primarily driven by the increasing number of patients who are initially ineligible for heart transplantation but may become eligible after stabilization with mechanical circulatory support, along with improved clinical outcomes and expanding use of VADs in complex heart failure management strategies.
- The market for the bridge-to-recovery (BTR) therapy segment is expected to expand rapidly, driven primarily by the increasing use of temporary mechanical circulatory support in post-cardiotomy and acute heart failure cases, where patients have a potential for myocardial recovery.
- On the other hand, the other applications segment held a market share of 5.6% in 2024, and its growth can be attributed to the increasing use of VADs in bridge-to-decision scenarios, post-cardiotomy support, and temporary circulatory stabilization, especially in patients with uncertain transplant eligibility or reversible cardiac conditions.
Based on the patient, the ventricular assist devices market is segmented into adults and pediatrics. The adults segment was anticipated to be worth USD 2.3 billion in 2024 and is expected to grow at an 8.2% CAGR during the forecast period.
- This growth is driven by the rising prevalence of advanced heart failure among aging populations, increasing adoption of destination therapy, and continuous technological advancements in device design and functionality.
- On the other hand, the market for the pediatrics segment is expected to grow at a 10.2% CAGR. This growth is primarily driven by the rising incidence of congenital heart defects, increasing demand for specialized pediatric cardiac care, and ongoing technological advancements in device miniaturization and biocompatibility tailored for younger patients.
Based on flow, the ventricular assist devices market is segmented into pulsatile flow and continuous flow. The continuous flow segment was anticipated to be worth USD 2.4 billion in 2024.
- Its dominance is further supported by widespread clinical adoption, lower complication rates, and strong long-term survival data across diverse patient populations. The segment is expected to maintain its lead due to ongoing innovations in pump design and energy efficiency.
- On the other hand, the market for the pulsatile flow segment is expected to grow at a 10.2% CAGR. Although less commonly used than continuous-flow devices, this segment is gaining traction due to its physiological mimicry of natural heartbeats, which can be beneficial in specific patient populations and recovery scenarios.
Based on design, the ventricular assist devices market is segmented into transcutaneous and implantable. The implantable segment was anticipated to be worth USD 2 billion in 2024.
- The strong preference for implantable VADs stems from their enhanced durability, improved patient outcomes, and technological advancements such as magnetic levitation pumps and wireless power systems. As the demand for long-term cardiac support continues to rise amid a growing heart failure population and limited donor availability, the implantable VAD segment is expected to remain a cornerstone of the market's growth trajectory.
- On the other hand, the market for the transcutaneous segment is expected to grow at a 8% CAGR. This growth is primarily driven by the increasing adoption of minimally invasive procedures, the expanding use of percutaneous micro-axial pumps, and the segment’s suitability for short-term cardiac support in high-risk PCI and cardiogenic shock cases.
Learn more about the key segments shaping this market
Based on end use, the ventricular assist devices market is segmented into hospitals, cardiac catheterization labs, ambulatory surgical centers, and other end users. The hospitals segment dominated the market with a revenue share of 43.1% in 2024 and is expected to reach USD 2.4 billion within the forecast period.
- The growth in this segment is driven by the increasing number of advanced heart failure cases requiring surgical intervention and post-operative care in hospital settings.
- Hospitals continue to be the primary centers for VAD implantation, monitoring, and long-term management, making them critical to the market’s sustained growth.
- Moreover, the availability of skilled cardiac surgeons and advanced infrastructure in hospitals further reinforces their leading role in VAD adoption.
- The cardiac catheterization labs segment was valued at approximately USD 723.5 million in 2024, driven by the rising prevalence of cardiovascular diseases, increasing demand for minimally invasive procedures, and continuous advancements in imaging technologies such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT).
- The market for the ambulatory surgical centers (ASCs) segment is expected to expand rapidly, driven primarily by the growing demand for minimally invasive and same-day procedures, favorable reimbursement policies, and the shift from inpatient to outpatient care models. ASCs offer cost-effective surgical solutions, reduced infection risks, and faster recovery times, making them increasingly attractive to both patients and healthcare providers.
- On the other hand, the other end users segment held a market share of 6.4% in 2024, and its growth can be attributed to the increasing adoption of VADs in specialty clinics and research institutions.

Looking for region specific data?
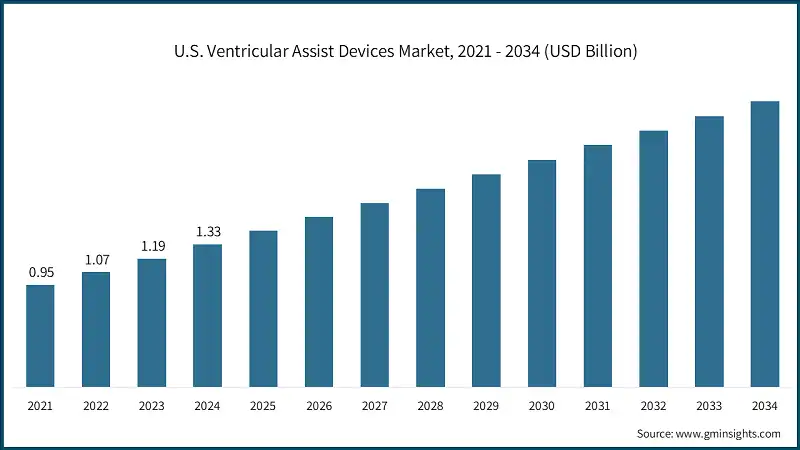
North America Ventricular Assist Devices Market North America dominated the global market with the highest market share of 57.4% in 2024. Europe market accounted for USD 821.4 million in 2024 and is anticipated to show lucrative growth over the forecast period. The Asia Pacific market is anticipated to grow at the highest CAGR of 13.7% during the analysis timeframe. The Latin America market is experiencing robust growth over the analysis timeframe. The Middle East and Africa (MEA) market is experiencing notable growth over the analysis timeframe. Leading industry players such as Abbott, Berlin Heart, CorWave, Jarvik HEART, and Johnson & Johnson hold around 93% of the market share in the highly consolidated market. These companies maintain their leading position by combining strong product lines, business collaborations with healthcare providers, regulatory clearances, and consistent product innovation. Abbott continues to lead the market with its flagship HeartMate 3 LVAD, which has demonstrated superior survival rates and reduced complications such as thrombosis and stroke. Abbott’s strength lies in its diverse cardiovascular portfolio, strategic acquisitions, and robust clinical data supporting its devices. The company’s focus on remote monitoring, AI-powered diagnostics, and minimally invasive technologies positions it as a dominant force in both developed and emerging markets. Berlin Heart specializes in pediatric and paracorporeal VADs, with its EXCOR Pediatric device being the only FDA-approved VAD for infants and children. The company’s focus on clinical support, customized therapy programs, and collaborations with pediatric cardiac centers has made it a trusted name in specialized heart failure treatment. Berlin Heart’s strategic expansion into North America and Europe continues to strengthen its market share. Meanwhile, other key players such as Johnson & Johnson, Berlin Heart and CorWave are reinforcing their market presence through specialized innovations and strategic expansion. A few of the prominent players operating in the ventricular assist devices industry include: Johnson & Johnson, through its Abiomed division, leads with the Impella heart pump platform, the world’s smallest heart pump device. The Impella CP, 5.5, and ECP models offer minimally invasive, temporary mechanical circulatory support, particularly for patients with cardiogenic shock and high-risk PCI. Abbott distinguishes itself in the VAD market through its advanced blood flow technologies, which prioritize hemocompatibility, reduced complications, and long-term durability. The company’s devices are known for their fully magnetically levitated systems, which minimize wear and tear and improve patient outcomes. Abbott also integrates remote monitoring capabilities and digital health platforms to support clinicians in managing patients post-implantation. Its strong clinical trial data and global regulatory approvals reinforce its reputation for safety and efficacy across diverse patient populations. Berlin Heart is recognized globally for its specialization in pediatric and customized mechanical circulatory support solutions. Its systems are designed to accommodate a wide range of patient sizes and clinical needs, particularly in infants and children with end-stage heart failure. The company emphasizes pulsatile flow technology, which closely mimics natural heart function, and collaborates extensively with pediatric cardiac centers worldwide.Europe Ventricular Assist Devices Market
Asia Pacific Ventricular Assist Devices Market
Latin America Ventricular Assist Devices Market
Middle East and Africa Ventricular Assist Devices Market
Ventricular Assist Devices Market Share
Ventricular Assist Devices Market Companies
Ventricular Assist Devices Industry News:
The ventricular assist devices market research report includes an in-depth coverage of the industry with estimates and forecasts in terms of revenue in USD Million and volume in Units from 2021 - 2034 for the following segments:
Market, By Product
- Left ventricular assist devices (LVADs)
- Right ventricular assist devices (RVADs)
- Biventricular assist devices (BIVADs)
- Percutaneous ventricular assist devices
Market, By Application
- Destination therapy
- Bridge-to-candidacy (BTC) therapy
- Bridge-to-transplant (BTT) therapy
- Bridge-to-recovery (BTR) therapy
- Other applications
Market, By Patient
- Adults
- Pediatrics
Market, By Flow
- Pulsatile flow
- Continuous flow
- Axial continuous flow
- Centrifugal continuous flow
Market, By Design
- Transcutaneous
- Implantable
Market, By End Use
- Hospitals
- Cardiac catheterization labs
- Ambulatory surgical centers
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
What are the upcoming trends in the ventricular assist devices industry?
Key trends include adoption of continuous-flow and fully implantable systems, integration of wireless monitoring, biocompatible materials, and digital health platforms for real-time patient management.
Which region leads the ventricular assist devices market?
North America held 57.4% share with USD 1.3 billion in 2024, fueled by high heart failure prevalence, advanced cardiac care infrastructure, and early adoption of innovative devices.
How much revenue did the left ventricular assist devices (LVADs) segment generate?
The LVADs segment accounted for 48.2% of the market in 2024 and is projected to exceed USD 2.6 billion by 2034.
What was the valuation of the percutaneous VADs segment?
The percutaneous ventricular assist devices segment held a 37.4% share in 2024, driven by demand for minimally invasive procedures.
Who are the key players in the ventricular assist devices market?
Key players include Abbott, Johnson & Johnson, Berlin Heart, Jarvik Heart, CorWave, BrioHealth, and EVAHEART.
What is the projected size of the ventricular assist devices industry in 2025?
The ventricular assist devices market is expected to reach USD 2.7 billion in 2025.
What is the projected value of the ventricular assist devices market by 2034?
The market is expected to reach USD 5.7 billion by 2034, supported by miniaturization, wireless systems, and growing adoption in emerging markets.
What is the market size of the ventricular assist devices in 2024?
The market size was USD 2.5 billion in 2024, with a CAGR of 8.4% expected through 2034 driven by increasing heart failure cases, technological advancements, and shortage of heart donors.
Ventricular Assist Devices Market Scope
Related Reports


