Summary
Table of Content

U.S. Molecular Diagnostics Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
U.S. Molecular Diagnostics Market Size
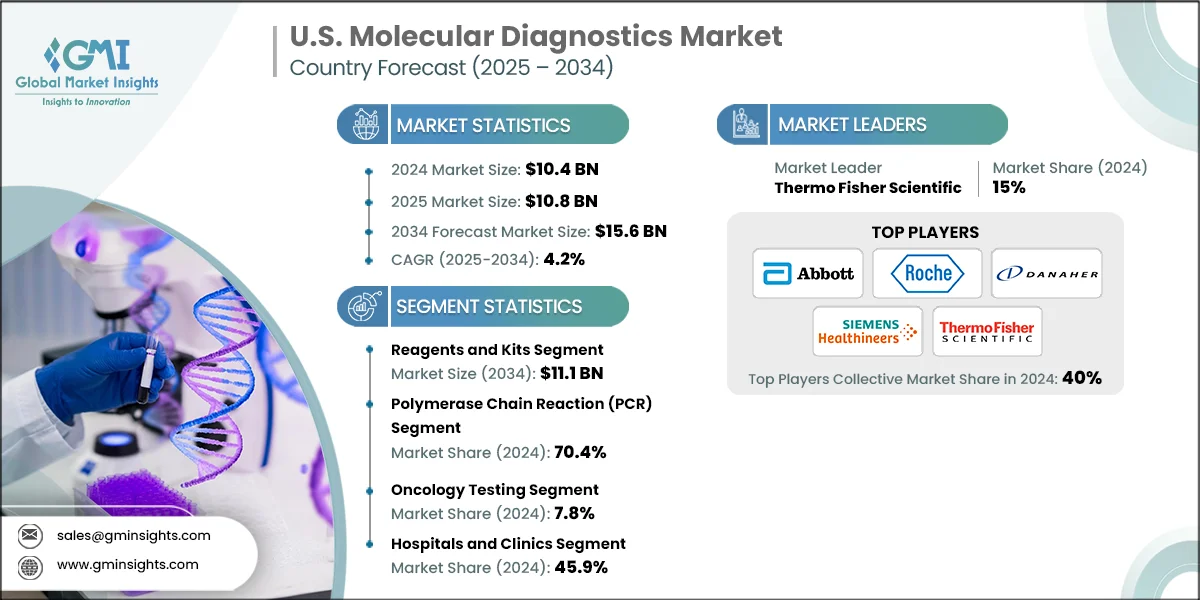
The U.S. molecular diagnostics market was valued at USD 10.4 billion in 2024 and is projected to grow from USD 10.8 billion in 2025 to USD 15.6 billion by 2034, expanding at a CAGR of 4.2%, according to the latest report published by Global Market Insights Inc. This steady growth is stimulated by various factors such as rising prevalence of chronic and infectious diseases, technological advancements in molecular diagnostics, rising government initiatives and healthcare spending, and increasing geriatric population base. Major companies in the industry include Abbott Laboratories, F. Hoffmann-La Roche, Danaher Corporation, Siemens Healthineers, and Thermo Fisher Scientific.
To get key market trends
The molecular diagnostics market has experienced significant growth, increasing from USD 8.8 billion in 2021 to USD 10.1 billion in 2023. This growth is primarily driven by the rising burden of chronic and infectious diseases, including COVID-19, influenza, RSV, tuberculosis, HIV, and hepatitis B and C. For instance approximately 1.2 million people in the U.S. are living with HIV, and an estimated 31,800 individuals acquired HIV in 2022. These figures highlight the ongoing need for reliable and accessible HIV diagnostic testing solutions to support early detection and reduce transmission. The increasing prevalence of HIV and other emerging health threats is accelerating the adoption of molecular diagnostics, further fueling market expansion.
U.S. Molecular Diagnostics Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 10.4 Billion |
| Market Size in 2025 | USD 10.8 Billion |
| Forecast Period 2025 - 2034 CAGR | 4.2% |
| Market Size in 2034 | USD 15.6 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising prevalence of chronic and infectious diseases | Drives demand for accurate and early diagnostic solutions, increasing test volumes and encouraging the adoption of advanced molecular platforms. |
| Increasing technological advancements in molecular diagnostics | Enhances test speed, accuracy, and accessibility, expanding market potential and enabling broader clinical applications. |
| Rising government initiatives and healthcare spending | Fuels innovation and the development of novel diagnostic tools, improving affordability and accelerating market penetration. |
| Growing geriatric population base | Boosts demand due to the higher susceptibility to chronic and infectious diseases. |
| Pitfalls & Challenges | Impact |
| High cost of molecular diagnostic tests and equipment | Limits accessibility and adoption, particularly in low-resource settings. |
| Stringent regulatory scenario | Slows product approvals and market entry, hindering innovation and delaying the commercialization of new technologies. |
| Opportunities: | Impact |
| Increasing AI and automation integration | Enables faster, more precise diagnostics and predictive insights, revolutionizing personalized medicine and improving operational efficiency in laboratories. |
| Market Leaders (2024) | |
| Market Leaders |
15% Market Share |
| Top Players |
Collective market share in 2024 is 40% |
| Competitive Edge |
|
| Regional Insights | |
| Future outlook |
|
What are the growth opportunities in this market?
Additionally, the rapidly growing elderly population in the U.S. is significantly contributing to this demand. Individuals aged 65 and older are more susceptible to chronic illnesses that require regular diagnostic testing. For instance, according to the Population Reference Bureau, the number of people in this age group is projected to rise from 58 million in 2022 to 82 million by 2050, marking an approximately 42% increase. Moreover, their share of the total population is expected to grow from 17% to 23% during the same period. As the proportion of older adults increases, so does the need for dependable diagnostic solutions to manage age-related conditions, further accelerating the growth of the molecular diagnostics market.
Molecular diagnostics refers to a diagnostic method that examines biological markers in the genome and proteome, including RNA, DNA, and proteins, to identify and monitor various diseases. This technique is widely used for diagnosing genetic disorders, infectious diseases, and cancer due to its high effectiveness and precision.
U.S. Molecular Diagnostics Market Trends
- Molecular diagnostics has become an important part of modern healthcare, helping doctors detect and understand diseases at the genetic and molecular levels with precision. These advancements have made diagnosis more accurate, reduced the time it takes to get results, and supported personalized treatment plans, ultimately improving patient care. With the increasing need for early disease detection and targeted therapies, the use of advanced molecular diagnostic tools has grown rapidly in areas such as cancer, infectious diseases, and genetic disorders.
- One of the most important technologies in this field is the polymerase chain reaction (PCR), which amplifies tiny amounts of DNA to make detection possible. Over the years, traditional PCR has advanced into more sophisticated versions such as real-time PCR (qPCR) and digital PCR, which offer greater sensitivity and the ability to measure results quantitatively. These improvements have made PCR an essential tool in both clinical and research settings, where it is widely used for detecting pathogens, analyzing cancer biomarkers, and screening for genetic conditions.
- For instance, Huwel Lifesciences’ RT-PCR system is a strong example of innovation in this space. This portable and compact molecular diagnostic device is designed for research, diagnostics, and field testing. It can process up to 16 samples, has fast ramp rates for time-sensitive workflows, requires no maintenance, and includes USB compatibility for easy data transfer. This demonstrates how technology is making molecular diagnostics more accessible and efficient.
- Additionally, the growing use of point-of-care molecular diagnostics, which is estimated to reach USD 90 billion by 2032, is revolutionizing healthcare delivery, especially in developing regions. By bringing diagnostic tools closer to patients, particularly in remote or resource-limited areas, point-of-care solutions enable timely medical interventions and reduce the strain on centralized laboratories.
U.S. Molecular Diagnostics Market Analysis
Learn more about the key segments shaping this market
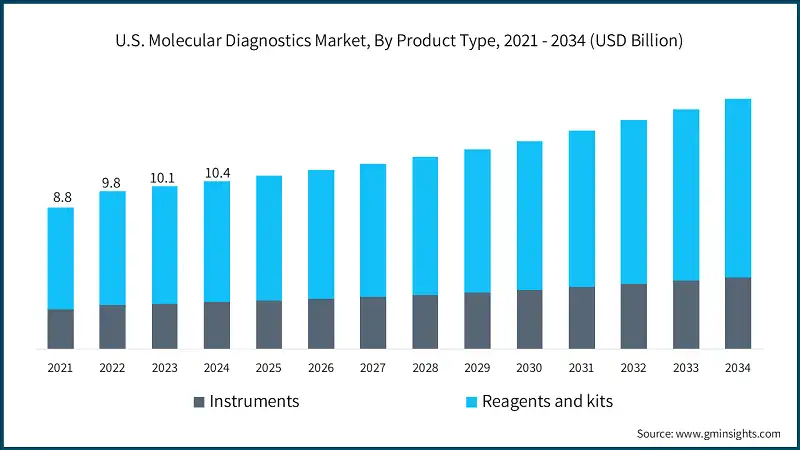
The market was valued at USD 8.8 billion in 2021. The market size reached USD 10.1 billion in 2023, from USD 9.8 billion in 2022.
Based on the product type, the market is segmented into instruments and reagents and kits. The reagents and kits segment led this market in 2024, accounting for the highest market share. This segment was valued at USD 7.5 billion in 2024 and is projected to reach USD 11.1 billion by 2034, growing at a CAGR of 4.1%. In comparison, the instruments segment was valued at USD 2.9 billion in 2024 and is projected to reach USD 4.5 billion by 2034, growing at a CAGR of 4.4%.
- Molecular diagnostics depends on a variety of specialized instruments that detect, amplify, and analyze nucleic acids and other biomolecules. These instruments form the foundation of modern diagnostic labs, making it possible to accurately detect diseases, perform genetic screening, and enable personalized medicine.
- These tools are essential for processing biological samples, identifying genetic material, and delivering precise diagnostic results. They are a critical part of molecular testing workflows and are widely used in clinical labs, hospitals, and research facilities.
- The rapid growth of this segment is fueled by ongoing technological advancements that improve speed, automation, and portability. Today’s instruments are designed to handle high-throughput tasks, feature integrated software for data analysis, and require minimal manual effort, making them more efficient and easier to use.
- For instance, the GeneXpert System by Cepheid is a molecular diagnostic tool designed for point-of-care use. This advanced platform uses real-time PCR technology to provide fast and highly accurate results for a wide range of infectious diseases, significantly reducing diagnostic turnaround times in decentralized healthcare settings.
- With their high usage rates and continuous technological improvements, instruments remain the driving force behind the growth of the molecular diagnostics market.
Based on technology, the molecular diagnostics market is segmented into polymerase chain reaction (PCR), hybridization, sequencing, isothermal nucleic acid amplification technology (INAAT), microarrays, and other technologies. The polymerase chain reaction (PCR) segment accounted for the highest market share of 70.4% in 2024.
- Polymerase chain reaction (PCR) is a widely used technique in molecular diagnostics. It helps amplify specific DNA or RNA sequences, making it possible to detect and analyze even smallest amounts of genetic material with high accuracy.
- Digital PCR (dPCR) takes PCR technology to the next level. Unlike traditional PCR, which measures amplification in bulk, dPCR splits a sample into thousands of individual reactions. This approach allows for precise detection of target sequences, even when they are present in very low concentrations.
- For example, the QIAcuity Digital PCR by QIAGEN is an advanced instrument designed for accurate and absolute quantification of nucleic acids using digital PCR technology. It uses nanoplates to divide samples into thousands of reactions, ensuring highly sensitive detection of rare targets without requiring calibration curves.
- Further, PCR is considered one of the most important tools in molecular diagnostics because it is both versatile and reliable. Continuous advancements in PCR technology, along with its widespread use in laboratories and hospitals, are key drivers of growth in the molecular diagnostics market.
Based on applications, the molecular diagnostics market is segmented into infectious disease, genetic disease testing, oncology testing, and other applications. The infectious disease diagnostics market is further bifurcated into, COVID-19, flu, respiratory syncytial virus (RSV), tuberculosis, CT/NG, HIV, Hepatitis C, Hepatitis B, and other infectious disease diagnostics. The oncology testing segment accounted for the market share of 7.8% in 2024.
- The increasing global prevalence of cancer is a major factor driving the growth of the molecular diagnostics segment. For instance, according to estimates from the Cancer Atlas, the global incidence of cancer is expected to rise by nearly 60% by 2040, reaching approximately 29.4 million new cases compared to 18.1 million cases reported in 2018.
- This growing cancer burden, driven by lifestyle changes, an aging population, and environmental factors, is fueling demand for advanced molecular diagnostic solutions. Instruments, kits, and reagents are becoming essential tools for early cancer detection and personalized treatment planning.
- Moreover, as the number of cancer cases continues to rise, healthcare systems are increasingly adopting advanced molecular diagnostics. The need for early detection, improved disease management, and public health monitoring is driving market growth and innovation in diagnostic technologies.
Learn more about the key segments shaping this market
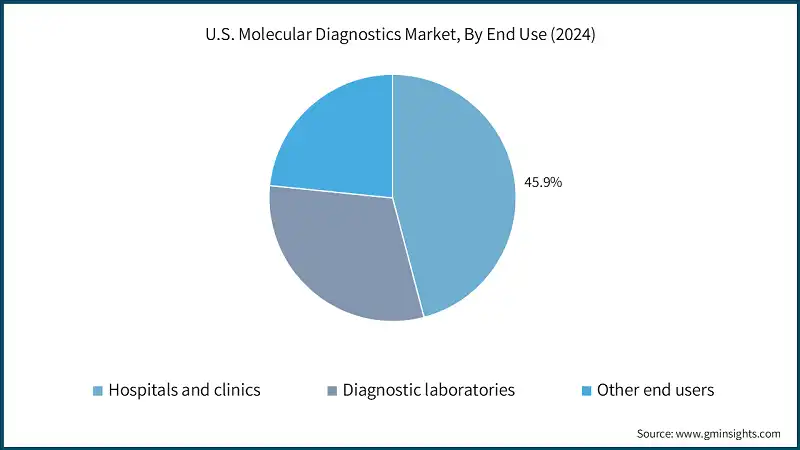
Based on end use, the molecular diagnostics market is segmented into hospitals and clinics, diagnostic laboratories, and other end users. The hospitals and clinics segment accounted for the highest market share of 45.9% in 2024.
- Hospitals play an important role in the molecular diagnostics market. They are equipped to handle high patient volumes and complex testing needs. With advanced laboratory infrastructure and skilled personnel, hospitals serve as key centers for conducting a wide range of molecular tests, from infectious disease screenings to cancer diagnostics.
- Hospitals seamlessly integrate molecular diagnostics into both routine care and emergency settings, ensuring consistent demand and utilization. Whether it is for early disease detection or rapid outbreak response, hospitals maintain a steady flow of diagnostic activities, making them essential in healthcare delivery.
- Additionally, hospitals often collaborate with research institutions and diagnostic companies to test new technologies and conduct clinical trials. These partnerships help accelerate the adoption of innovative molecular platforms and position hospitals as leaders in diagnostic innovation.
- Thus, the hospital segment continues to drive growth in the molecular diagnostics market through its extensive testing capabilities and integration with advanced technologies.
U.S. Molecular Diagnostics Market Share
- Major players such as Abbott Laboratories, F. Hoffmann-La Roche, Danaher Corporation, Siemens Healthineers, and Thermo Fisher Scientific collectively account for 40% of the U.S. molecular diagnostics market share. Their leadership is driven by extensive product portfolios, strategic partnerships, regulatory approvals, and continuous innovation. Among these, Thermo Fisher Scientific stands out for its diverse range of diagnostic solutions, which are widely adopted across various healthcare settings, providing it with a competitive edge.
- Becton, Dickinson and Company has established a strong position in the market through strategic collaborations and product launches. For instance, in June 2022, Becton, Dickinson and Company partnered with CerTest Biotec to develop diagnostic tests for detecting the monkeypox virus. This collaboration allowed the company to expand its product offerings and enhance its research capabilities.
- Emerging players in the U.S. molecular diagnostics space are adopting multi-faceted strategies to gain market traction. These strategies include focusing on advanced technologies such as PCR, next-generation sequencing (NGS), AI-powered analytics, and point-of-care platforms. Many are targeting areas like personalized medicine, particularly in oncology, while forming strategic alliances with hospitals, research institutions, and established firms to address high equipment costs and regulatory challenges.
U.S. Molecular Diagnostics Market Companies
Few of the prominent players operating in the U.S. molecular diagnostics industry include:
- Abbott Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Biocartis
- bioMérieux
- Bio-Rad Laboratories
- Danaher Corporation
- F. Hoffmann-La Roche
- Hologic
- Illumina
- Qiagen
- QuidelOrtho Corporation
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific
- F. Hoffmann-La Roche
F. Hoffmann-La Roche is a leading player in molecular diagnostics, offering advanced PCR-based assays, sequencing platforms, and companion diagnostics for oncology, infectious diseases, and genetic testing. With innovations and strong R&D investments, Roche drives personalized healthcare and integrated diagnostic solutions, reinforcing its market leadership.
Danaher Corporation employs a robust global workforce of approximately 63,000 individuals, which supports the company's ability to innovate and deliver high-quality solutions effectively.
Abbott Laboratories maintains a robust product portfolio, driving widespread adoption and contributing to significant market growth. The company offers a range of point-of-care testing products, including the Abbott Panbio COVID-19 Antigen Rapid Test, among others.
U.S. Molecular Diagnostics Industry News
- In May 2022, Abbott received FDA clearance for its Alinity m STI Assay, which detects four common STIs CT, NG, TV, and MG, from a single swab or urine sample. Designed for the Alinity m system, the assay uses highly sensitive PCR technology to deliver accurate results, improving diagnostic efficiency and expanding Abbott’s molecular diagnostics portfolio.
The U.S. molecular diagnostics market research report includes in-depth coverage of the industry with estimates and forecasts in terms of revenue in USD Million and from 2021 - 2034 for the following segments:
Market, By Product Type
- Instruments
- Reagents and kits
Market, By Technology
- Polymerase chain reaction (PCR)
- Hybridization
- Sequencing
- Isothermal nucleic acid amplification technology (INAAT)
- Microarrays
- Other technologies
Market, By Application
- Infectious disease diagnostics
- COVID-19
- Flu
- Respiratory syncytial virus (RSV)
- Tuberculosis
- CT/NG
- HIV
- Hepatitis C
- Hepatitis B
- Other infectious disease diagnostics
- Genetic disease testing
- Oncology testing
- Other applications
Market, By End Use
- Hospitals and clinics
- Diagnostic Laboratories
- Other end use
Frequently Asked Question(FAQ) :
Who are the key players in the U.S. molecular diagnostics market?
Major companies include Abbott Laboratories, F. Hoffmann-La Roche, Danaher Corporation, Siemens Healthineers, Thermo Fisher Scientific, Hologic, Illumina, Qiagen, QuidelOrtho, Sysmex Corporation, Bio-Rad Laboratories, Agilent Technologies, Biocartis, and bioMérieux.
What are the upcoming trends in the U.S. molecular diagnostics market?
Key trends include adoption of AI-driven diagnostic workflows, advanced automation, digital PCR expansion, point-of-care molecular solutions, and increased use of sequencing-based testing.
What is the growth outlook for the reagents & kits segment from 2025 to 2034?
The reagents & kits segment is expected to grow at a 4.1% CAGR through 2034, driven by continuous demand for molecular testing across infectious disease, oncology, and genetic applications.
What was the valuation of the instruments segment in 2024?
The instruments segment was valued at USD 2.9 billion in 2024, supported by increased adoption of automated and high-throughput diagnostic platforms.
What is the market size of the U.S. molecular diagnostics industry in 2024?
The market size was USD 10.4 billion in 2024, with a CAGR of 4.2% expected through 2034, supported by rising prevalence of chronic and infectious diseases.
How much revenue did the reagents & kits segment generate in 2024?
The reagents & kits segment generated USD 7.5 billion in 2024, holding the largest share due to high testing volume and recurring consumable demand.
What is the current U.S. molecular diagnostics market size in 2025?
The market size is projected to reach USD 10.8 billion in 2025.
What is the projected value of the U.S. molecular diagnostics market by 2034?
The industry is expected to reach USD 15.6 billion by 2034, driven by technological advancements and growing demand for early and precise diagnostic testing.
U.S. Molecular Diagnostics Market Scope
Related Reports


