Summary
Table of Content

Thermoplastic Elastomers in Medical Devices Market
Get a free sample of this report
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Thermoplastic Elastomers in Medical Devices Market Size
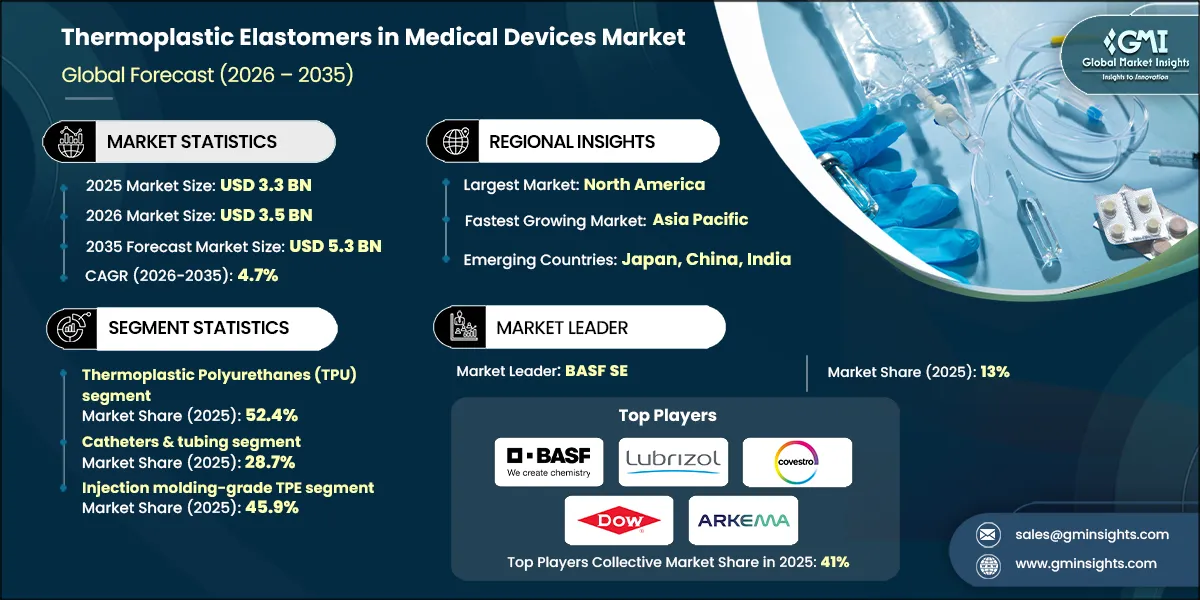
The global thermoplastic elastomers in medical devices market was valued at USD 3.3 billion in 2025. The market is expected to grow from USD 3.5 billion in 2026 to USD 5.3 billion in 2035, at a CAGR of 4.7% according to latest report published by Global Market Insights Inc.
To get key market trends
- The development of thermoplastic elastomers has transformed these materials from specialized functions into essential components for creating contemporary medical devices. Their inherent properties of flexibility and biocompatibility and processability make them essential materials for various clinical uses. The healthcare industry now requires devices that offer both long-lasting performance and comfortable patient experience which makes TPEs crucial for designing upcoming products that include catheters and wearable devices and seals and soft-touch instrument elements and drug delivery systems. The sector now utilizes materials which improve functional capabilities while enabling advanced treatment methods that focus on patient needs.
- The TPE ecosystem for medical use has established sustainability and material stewardship as its core principles. The regulatory drive for material safety and environmental cleanliness and compliance with regulations is increasing demand for TPEs which provide recyclability and lower environmental effects and meet strict sterilization standards. Manufacturers now emphasize material innovation to address legacy issues which stem from traditional elastomers including latex allergies and plasticizer safety problems. The industry conducts permanent environmental and clinical safety evaluations which show its progress toward better formulation science results that enhance product strength and chemical durability and service life.
- The medical device value chain now receives stronger TPE support through technological advances. The advancements in extrusion and injection molding and multi-component processing enable manufacturers to produce custom geometries and high-precision components and complete device systems that simplify production procedures.
Thermoplastic Elastomers in Medical Devices Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2025 |
| Market Size in 2025 | USD 3.3 Billion |
| Market Size in 2026 | USD 3.5 Billion |
| Forecast Period 2026-2035 CAGR | 4.7% |
| Market Size in 2035 | USD 5.3 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Shift from PVC to phthalate-free alternatives | Manufacturers now prefer TPE materials because they provide safer options which help them achieve faster regulatory approval |
| Growing demand for latex-free medical products | Provide better patient protection and comfort. |
| Aging population & chronic disease prevalence | Medical industry uses TPE materials for producing catheters and tubing and wearables because these items serve long-term care and minimally invasive procedures |
| Pitfalls & Challenges | Impact |
| Higher material cost vs. Commodity PVC | Economic pressure in price-sensitive device categories where low-cost PVC still dominates |
| Limited high-temperature resistance vs. Thermosets | Thermosets offer better performance than TPEs in high-heat environments and extreme-condition settings |
| Opportunities: | Impact |
| Sustainable & bio-based tpe formulations | Requires companies to produce eco-friendly TPE products which help them achieve environmental standards and reduce compliance expenses |
| Antimicrobial & infection-prevention materials | Become essential to medical applications which include disposable products and wearable devices and hospital equipment |
| Market Leaders (2025) | |
| Market Leader |
13% |
| Top Players |
Collective Market Share of 41% in 2025 |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | Japan, China, India |
| Future outlook |
|
| Companies covered: | 15 |
| Tables, Charts & Figures: | 205 |
| Countries covered: | 18 |
| No of Pages: | 190 |
What are the growth opportunities in this market?
Thermoplastic Elastomers in Medical Devices Market Trends
- Thermoplastic elastomers in medical devices have rapidly evolved from niche flexible polymers into foundational enablers of next-generation healthcare technologies. The combination of biocompatibility and sterilization stability together with rubber-like elasticity and thermoplastic processability creates their medical application compatibility range which includes tubing and catheters and seals and wearable interfaces and ergonomic surgical components. The sector demands new materials which deliver enhanced performance and durability and patient protection from increasingly strict regulatory requirements. The development of TPEs into essential device design materials shows their transition from basic elastomeric options to crucial resources which maintain essential functions in contemporary medical systems.
- The medical-grade TPE market is undergoing transformation through new standards which emerge from rising sustainability objectives and regulatory demands for safer and cleaner product formulations. The material developers are focusing on creating elastomer systems which do not contain solvents or latex or PVC yet display higher biostability and recyclability and meet all global medical standards. The production process improvements which include high-precision extrusion and co-injection overmolding and multi-layer manufacturing enable manufacturers to achieve better material efficiency and performance consistency while reducing waste. The healthcare device sector adopts these advanced developments to create circular economy systems which make medical TPEs future-proof materials that support innovation through sustainable environmental solutions.
- Thermoplastic elastomers will gain more strategic value in the future because medical device technologies will connect with digital health systems and minimally invasive treatments and home-based medical care and connected wearable devices. TPEs function as essential components for upcoming medical technologies which need to achieve human-centered design standards while maintaining clinical environment requirements. TPEs will drive industry innovation because their lightweight design enables device manufacturers to achieve performance and regulatory and sustainability goals which healthcare markets worldwide need.
Thermoplastic Elastomers in Medical Devices Market Analysis
Learn more about the key segments shaping this market
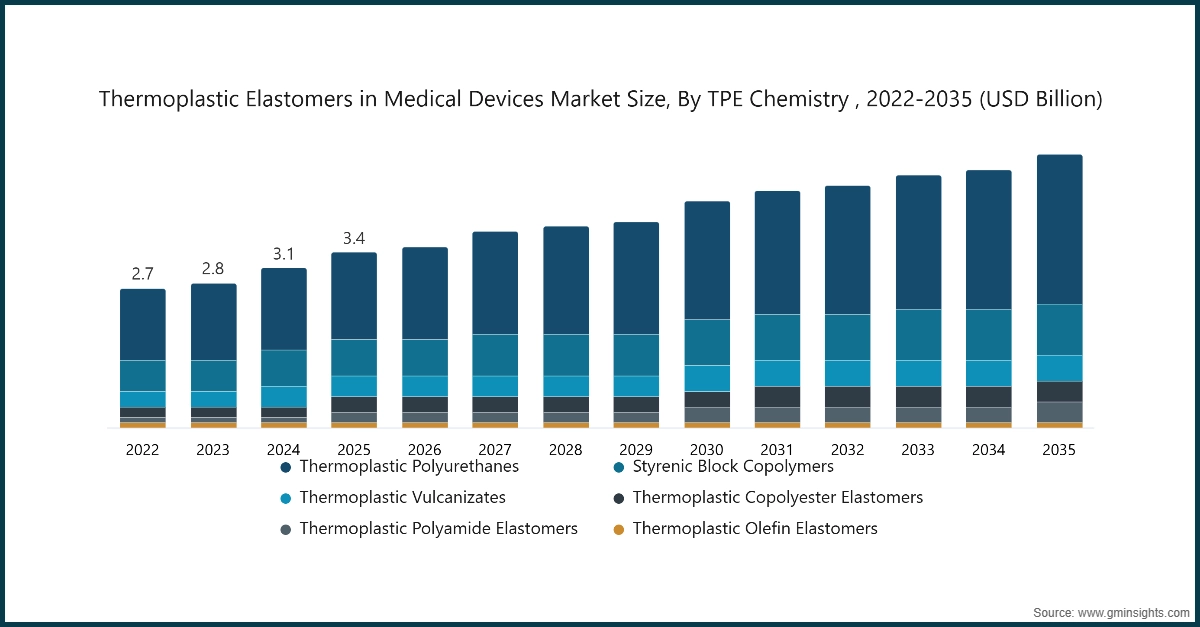
Based on TPE chemistry, the market is segmented into Thermoplastic Polyurethanes (TPU), Styrenic Block Copolymers (TPE-S/SEBS), Thermoplastic Vulcanizates (TPV), Thermoplastic Copolyester Elastomers (COPE/TPC-ET), Thermoplastic Polyamide Elastomers (PEBA/TPE-A), Thermoplastic Olefin Elastomers (TPE-O/TPO). Thermoplastic Polyurethanes (TPU) dominated the market with an approximate market share of 52.4% in 2025 and is expected to grow with a CAGR of 5% by 2035.
- Thermoplastic Polyurethanes TPU the most vital medical-grade TPE material because these properties enable manufacturers to create advanced medical tubing and catheters and films and wearable interfaces. TPU material behavior enables design engineers to develop products with controlled softness and structural integrity which allows the operation of minimally invasive tools and fluid management systems.
- The medical field now recognizes several important materials which include Styrenic Block Copolymers TPE-S/SEBS and Thermoplastic Vulcanizates TPV and Thermoplastic Copolyester Elastomers COPE/TPC-ET and Thermoplastic Polyamide Elastomers PEBA/TPE-A and Thermoplastic Olefin Elastomers TPE-O/TPO for their ability to meet specific performance requirements needed in different medical use cases. The SEBS-based elastomers provide pure and soft materials which maintain stable processing properties, making them suitable for seals and grips and sensitive patient contact components, while PEBA materials which possess kink resistance and fatigue strength and lightweight performance make them perfect for developing next-generation catheter systems.
Learn more about the key segments shaping this market
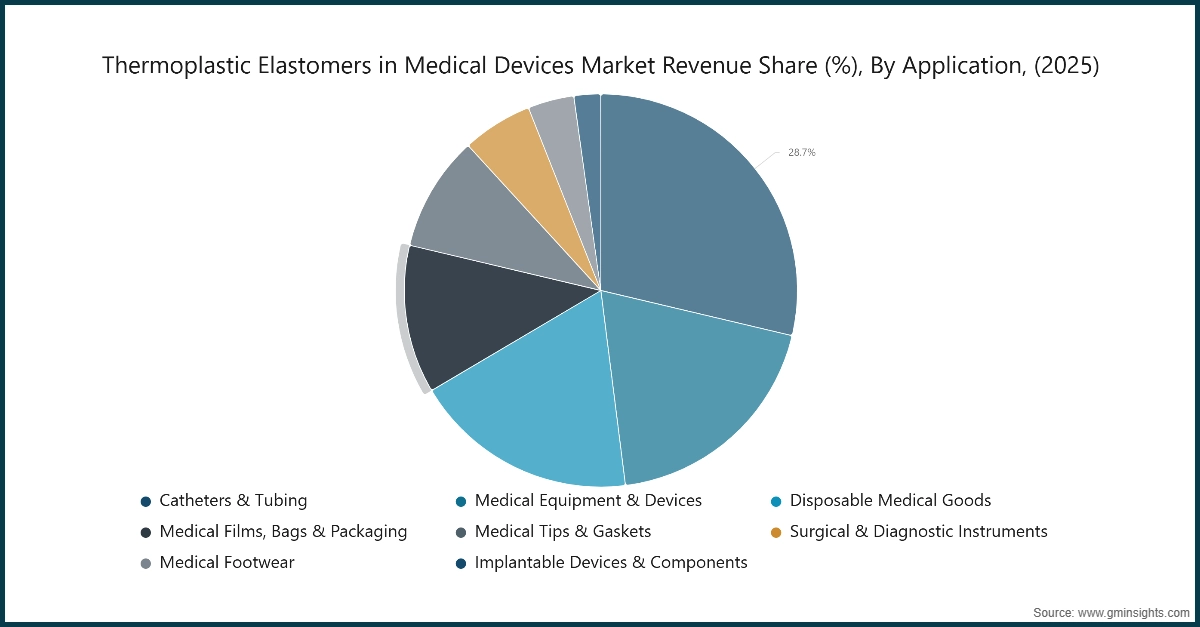
Based on application, the thermoplastic elastomers in medical devices market is segmented into catheters & tubing, medical equipment & devices, disposable medical goods, medical films, bags & packaging, medical tips & gaskets, surgical & diagnostic instruments, medical footwear, implantable devices & components. Catheters & tubing held the largest market share of 28.7% in 2025 and is expected to grow at a CAGR of 5.5% during 2026-2035.
- Catheters and tubing dominate the industry because these products require their essential biocompatibility and flexibility and ability to maintain sterilization throughout their lifespan. The material flexibility of the product enables it to function properly in all types of medical equipment and diagnostic devices and disposable items and seals and tips and films and specialized packaging, which helps maintain device dependability during standard medical procedures and advanced therapeutic applications.
- The development of wearable health technologies and implant- adjacent components shows that high-functioning TPE chemistries enable design progress by providing the essential mechanical performance and patient comfort that must remain consistent throughout this process. The medical sector needs TPE-based solutions to create more ergonomic devices which require less invasive procedures. The industry requires TPE-based solutions because they provide manufacturers with the necessary flexibility to adapt to changing clinical needs and regulatory requirements between various uses.
Based on processing method, the thermoplastic elastomers in medical devices market is segmented into injection molding-grade TPE, extrusion-grade TPE, blow molding-grade TPE, overmolding & co-injection grade TPE. Injection molding-grade TPE segment dominated the market with an approximate market share of 45.9% in 2025 and is expected to grow with the CAGR of 5.3% by 2035.
- Medical devices use injection molding-grade thermoplastic elastomers as their main processing method because these materials produce patient-contact parts that require tight specifications and their surfaces maintain consistent quality while their biocompatibility properties remain intact. The compatibility of these materials with high-volume manufacturing and multi-material parts production and tight-tolerance geometric design enables their use in ergonomic grips and device housings and soft-touch interfaces and catheter connectors and wearable system components which require design flexibility and compliance with regulatory standards.
- Medical applications currently use extrusion-grade TPE formulations and blow-molding-grade TPE formulations and overmolding/co-injection TPE formulations because these formulations provide specific benefits which include maintaining consistent tubing profiles and producing thin-wall films and providing reliable bonding strength for hybrid component assembly. The processing methods enable the development of modern health technologies and packaging components and wearable health technologies and precise seals which demonstrate TPEs' capacity to function as environmentally friendly materials which adapt to different needs while focusing on patient needs.
Looking for region specific data?
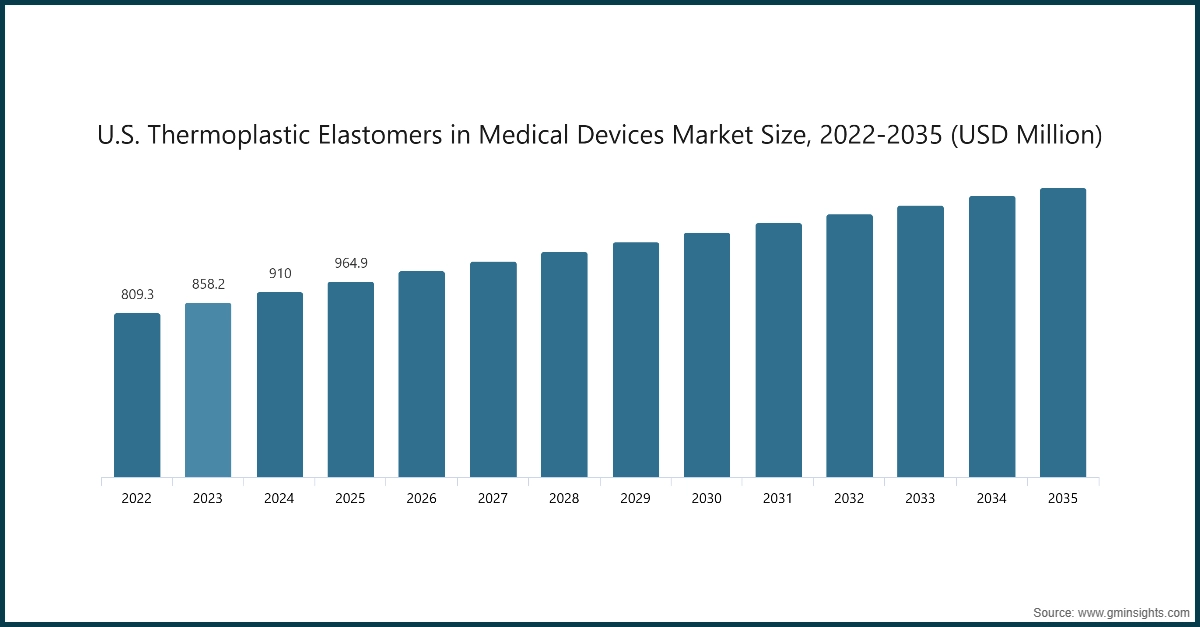
The North America thermoplastic elastomers in medical devices dominates the industry and growing rapidly on the global level with a market share of 35% in 2025.
- The North American region operates as a central Thermoplastic Elastomers hub for Medical Devices because its advanced healthcare system and strong regulatory framework and existing network of medical device producers who use high-performance elastomeric materials sustain their operations. The region maintains its position as the leading authority through its specialized knowledge of biocompatible polymers that meet sterilization requirements and its ongoing research activities which improve device safety standards and operational capabilities and patient experience.
U.S. dominates the North America thermoplastic elastomers in medical devices market, showcasing strong growth potential.
- The U.S. continues to drive technical progress because its medical technology sector and its established regulatory system and its fast-growing market for flexible durable TPEs used in minimally invasive devices and catheters and wearables and seals and drug delivery systems all exist within its borders.
Europe thermoplastic elastomers in medical devices market growing in the industry with revenue of USD 931.6 million in 2025 and is anticipated to show lucrative growth over the forecast period.
- The European continent functions as a crucial strategic area for Thermoplastic Elastomers in Medical Devices because its strict regulations and EU environmental safety standards force manufacturers to use environmentally friendly materials which are both safe and sustainable. The healthcare and medical device industry in this region demands biocompatibility and sterilization stability and environmentally friendly polymer solutions which automatically lead to the use of advanced medical grade TPEs in applications including tubing and catheters and seals and diagnostic components and wearable health solutions.
The Asia Pacific thermoplastic elastomers in medical devices market is anticipated to grow rapidly at a CAGR of 6.1% during the analysis timeframe.
- The growth of medical-device manufacturing in Asia-Pacific together with its clinical infrastructure modernization initiatives establishes the region as a global center for developing next-generation elastomeric solutions.
- The region's momentum receives its primary driving force from the rising importance of local production capabilities which create faster development cycles and stronger supply chain resilience and more precise biocompatible TPE component quality control. The healthcare providers and device manufacturers in Asia-Pacific require more advanced elastomer materials because they are implementing multi-material assemblies and wearable patient-monitoring devices and precision fluid-management systems into their design architectures.
Latin America thermoplastic elastomers in medical devices accounted for 4.2% market share in 2025 and is anticipated to show highest growth over the forecast period.
- The medical device production capabilities have progressed to a point where now create a need for TPEs that possess flexible and durable properties, while also meeting sterilization standards, which are required for their use in tubing and catheters and diagnostic components, as well as patient contact interfaces. The current medical materials momentum demonstrates a pattern that reflects how healthcare providers select safer polymers and more adaptable polymers as their preferred choice for both disposable and semi-durable clinical solutions.
Middle East & Africa thermoplastic elastomers in medical devices accounted for 2.8% market share in 2025 and is anticipated to show lucrative growth over the forecast period.
- Middle East and Africa region is growing because there is increased healthcare spending on modern medical facilities and people are starting to use biocompatible high-performance materials. The regional medical system upgrades raise demand for TPEs which offer flexible and durable properties and can be sterilized, these materials will be used to create medical products such as tubing and catheters and diagnostic components and wearable interfaces and patient contact parts.
Thermoplastic Elastomers in Medical Devices Market Share
The top 5 companies in Thermoplastic Elastomers in Medical Devices industry include BASF SE, Lubrizol Corporation, Covestro AG, Dow Chemical, Arkema. These are prominent companies operating in their respective regions covering approximately 41% of the market share in 2025. These companies hold strong positions due to their extensive experience in thermoplastic elastomers in medical devices market. Their diverse product portfolios, backed by robust production capabilities and distribution networks, enable them to meet the rising demand across various regions.
BASF SE functions as an international chemical enterprise which produces chemicals and materials and industrial solutions and surface technologies and nutrition and care products and agricultural solutions. The company provides products to multiple industries while maintaining international operations through its worldwide network of manufacturing facilities and research partnerships.
Lubrizol Corporation operates as a specialty chemical company which supplies transportation and industrial and consumer markets with its products. The company's product range includes engine oil additives and industrial lubricant additives and fuel additives and personal care and pharmaceutical materials and specialty application materials.
Covestro AG creates and produces advanced polymer materials which serve as the basis for making polyurethanes and coatings and adhesives and polycarbonates and specialty chemicals. The organization runs research laboratories and sales networks which serve customers in automotive construction electronics and healthcare industries throughout the world.
Dow Inc. delivers materials science solutions for packaging and infrastructure and mobility and consumer markets. The company produces multiple product categories which include plastics and intermediates and coatings and specialty materials.
Arkema provides specialized materials which include adhesive solutions and advanced materials and coating solutions and intermediates. The company's portfolio supports applications in construction, automotive, consumer goods, electronics and industrial sectors.
Thermoplastic Elastomers in Medical Devices Market Companies
Major players operating in the thermoplastic elastomers in medical devices industry include:
- BASF SE
- Lubrizol Corporation
- Covestro AG
- Dow Chemical
- Arkema
- Teknor Apex
- Kraiburg TPE
- Hexpol/Elastron
- Kuraray
- RTP Company
- Evonik
- Kraton
- DSM Biomedical
- Trinseo
- Mitsubishi Chemical
Thermoplastic Elastomers in Medical Devices Industry News
- In October 2022, Freudenberg Medical L.L.C., a segment of the German global technology business Freudenberg Group, offered HelixFlex, a new high-purity TPE tubing for fluid transfer in biopharmaceutical applications in response to growing demand for medical applications.
- In September 2021, DuPont inaugurated of its medical elastomers mixers at the Healthcare Industries Materials Site (HIMS) in Hemlock, Michigan, USA. The investment in the new mixers responds to increased supply needs from DuPont Liveo Healthcare Solutions customers.
This thermoplastic elastomers in medical devices market research report includes in-depth coverage of the industry, with estimates & forecasts in terms of revenue (USD Billion) and volume (Kilo Tons) from 2022 to 2035, for the following segments:
Market, By TPE Chemistry
- Thermoplastic Polyurethanes (TPU)
- Polyether-Based TPU
- Polyester-Based TPU
- Styrenic Block Copolymers (TPE-S/SEBS)
- SEBS (Hydrogenated Styrenic)
- SBS & Other Styrenic Variants
- Thermoplastic Vulcanizates (TPV)
- PP/EPDM Dynamic Vulcanizates
- Thermoplastic Copolyester Elastomers (COPE/TPC-ET)
- Polyether Glycol/PBT Block Copolymers
- Thermoplastic Polyamide Elastomers (PEBA/TPE-A)
- Thermoplastic Olefin Elastomers (TPE-O/TPO)
Market, By Application
- Catheters & tubing
- Intravenous (iv) catheters
- Urological catheters
- Cardiovascular catheters & balloon catheters
- Single & multi-lumen medical tubing
- Drug delivery & infusion tubing
- Medical equipment & devices
- Dental guards for bruxism disease
- Peristaltic pump tubes
- Urine catheter grips & components
- Respiratory face masks & seals
- Disposable medical goods
- Throat swab brushes
- Examination gloves (non-latex)
- Disposable masks & garments
- Medical films, bags & packaging
- IV & saline bags
- Biopharmaceutical storage bags
- Enteral & parenteral nutrition bags
- Peritoneal & dialysis bags
- Medical tips & gaskets
- Infusion bottle caps & closures
- Syringe gaskets & plunger tips
- Vial stoppers & septa
- Surgical & diagnostic instruments
- Surgical instrument grips & handles
- Diagnostic equipment housings
- Medical footwear
- Implantable devices & components
- Surgical meshes (polymeric)
- Polymeric prostheses components
- Elastomeric implant blocks
Market, By Processing Method
- Injection molding-grade TPE
- Extrusion-grade TPE
- Blow molding-grade TPE
- Overmolding & co-injection grade TPE
The above information is provided for the following
regions and countries:
· North America
- U.S.
- Canada
· Europe
- Germany
- UK
- France
- Spain
- Italy
- Rest of Europe
· Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Rest of Asia Pacific
· Latin America
- Brazil
- Mexico
- Argentina
- Rest of Latin America
· Middle East and Africa
- Saudi Arabia
- South Africa
- UAE
- Rest of Middle East and Africa
Frequently Asked Question(FAQ) :
Who are the key players in the thermoplastic elastomers in medical devices market?
Key players include BASF SE, Lubrizol Corporation, Covestro AG, Dow Chemical, Arkema, Teknor Apex, Kraiburg TPE, Hexpol/Elastron, Kuraray, RTP Company, Evonik, and Kraton.
What are the upcoming trends in the thermoplastic elastomers in medical devices industry?
Key trends include the development of sustainable and bio-based TPE formulations, increasing use of antimicrobial materials, and advancements in TPE solutions for wearable devices and drug delivery systems. The focus on eco-friendly and infection-prevention materials is also shaping the market.
Which region leads the thermoplastic elastomers in medical devices market?
North America led the market with a 35% share in 2025. Its dominance is driven by advanced healthcare infrastructure, high adoption of innovative medical technologies, and significant R&D investments in biocompatible materials.
What is the growth outlook for the injection molding-grade TPE segment from 2026 to 2035?
The injection molding-grade TPE segment is projected to grow at a CAGR of 5.3% through 2035. Its growth is supported by its widespread use in manufacturing medical devices due to its precision and cost-effectiveness.
What was the valuation of the catheters & tubing segment in 2025?
The catheters & tubing segment held the largest market share of 28.7% in 2025. Its dominance is driven by the growing need for long-term care solutions and minimally invasive procedures.
What was the market share of Thermoplastic Polyurethanes (TPU) in 2025?
Thermoplastic Polyurethanes (TPU) dominated the market with a 52.4% share in 2025. Its leadership is attributed to its superior biocompatibility and versatility in medical applications such as catheters, tubing, and wearable devices.
What is the projected value of the thermoplastic elastomers in medical devices market by 2035?
The market size for thermoplastic elastomers in medical devices is expected to reach USD 5.3 billion by 2035, growing at a CAGR of 4.7%. This growth is fueled by the aging population, increased prevalence of chronic diseases, and the demand for minimally invasive medical devices.
What is the market size of the thermoplastic elastomers in medical devices industry in 2026?
The market size for thermoplastic elastomers in medical devices reached USD 3.5 billion in 2026, reflecting steady growth supported by rising demand for latex-free medical products and advancements in healthcare technologies.
What is the thermoplastic elastomers in medical devices market size in 2025?
The market size for thermoplastic elastomers in medical devices was valued at USD 3.3 billion in 2025. The market's growth is driven by the increasing adoption of biocompatible materials and the shift from PVC to phthalate-free alternatives.
Thermoplastic Elastomers in Medical Devices Market Scope
Related Reports


