Summary
Table of Content

Peptide Synthesis Reagents Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Peptide Synthesis Reagents Market Size
The global peptide synthesis reagents market was valued at USD 729.9 million in 2024 and is projected to expand from USD 788.4 million in 2025 to USD 1.5 billion by 2034, reflecting a 7.4% CAGR from 2025 to 2034, according to latest report published by Global Market Insights Inc.
To get key market trends
- Fmoc-based and carbodiimide-based coupling reagents show the strongest demand trends, while uronium formulations and hybrid combination systems provide the pathway for broader market applications. The combination of improved bioavailability for peptide drug development and bio-based reagent alternatives with robust funding support for automated synthesis research and steady commercialization of green chemistry technologies explains this momentum. The FDA's peptide regulatory framework along with maturing precision medicine adoption in pharmaceutical, biotechnology, and diagnostic sectors further enhance progress in commercial applications.
- Peptide synthesis reagents are now emerging in the personalized medicine market that rapidly transforms into commercial-scale installations and advanced therapeutic peptide processing. Fmoc-based and solid-phase synthesis technologies demonstrate excellent scalability and efficacy balance, positioning them for next-generation drug development and bioactive peptide production operations. The cutting-edge synthesis capabilities of new reagent formulations with their seamless integration for pharmaceutical applications make these systems ideal for next-generation therapeutic development and precision peptide manufacturing production systems.
- The remarkable advancements in peptide-specific reagent design and bioactive component processing now make them more efficient, scalable, and greatly extended in terms of applications with wider acceptance of advanced synthesis systems for specialty pharmaceutical operations. Success in personalized peptide technologies and targeted therapeutic development methods yields better and more consistent clinical outcomes, while robust commercial-grade systems have improved performance in large-scale manufacturing production. This opens up the entire opportunity landscape for advanced commercialization, moving peptide synthesis reagents from research installations into market applications, modernizing traditional drug development and integrated precision medicine.
- The emerging trend towards personalized therapeutic solutions and advanced peptide drug production is gradually translating into substantial demand increases for peptide synthesis reagents in commercial pharmaceutical and precision medical applications. Advanced Fmoc processing systems, targeted synthesis platforms, and personalized reagent programs have become standard production technologies in commercial facilities, while novel processing systems and integrated automation showcase the variety of possible applications. Clear trends have emerged related to next-generation precision medicine facilities and therapeutic development systems that require high-performance biomarker-driven and peptide synthesis reagent processing technologies.
Peptide Synthesis Reagents Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 729.9 Million |
| Market Size in 2025 | USD 788.4 Million |
| Forecast Period 2025 – 2034 CAGR | 7.4% |
| Market Size in 2034 | USD 1.5 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing demand for peptide therapeutics and drug development solutions | Drives adoption of Fmoc-based reagents and coupling formulations for next-generation pharmaceutical development |
| Advances in solid-phase peptide synthesis and improved bioactive component identification | Enables broader applications from research facilities to commercial-scale peptide synthesis systems |
| Growing regulatory support for peptide drugs and investment in pharmaceutical research | Accelerates development of novel synthesis formulations for emerging therapeutic applications |
| Rising investment in biotechnology and precision medicine development | Encourages commercialization of advanced synthesis solutions for traditional and targeted pharmaceutical production |
| Pitfalls & Challenges | Impact |
| High development costs and complex formulation requirements | Limits adoption in cost-sensitive applications, requiring continued process optimization and cost reduction solutions |
| Performance validation compared to conventional synthesis methods | Extends development timelines and increases market entry barriers for new peptide synthesis technologies |
| Competition from recombinant production methods | Impacts market penetration in conventional applications, driving need for clear efficacy demonstration |
| Opportunities: | Impact |
| Expansion into clinical pharmaceutical and therapeutic applications | Enables market growth into high-value medical and pharmaceutical reagent production |
| Development of peptide-specific formulations for targeted therapeutic outcomes | Creates new market segments and increases value proposition for peptide synthesis reagent manufacturers |
| Integration into emerging technologies like automated synthesis and precision medicine | Opens new application areas in next-generation pharmaceutical and digital drug development |
| Strategic partnerships for co-development of personalized synthesis solutions | Accelerates market penetration through integrated design and pharmaceutical approaches |
| Market Leaders (2024) | |
| Market Leaders |
Market Share Approximately 14% |
| Top Players |
Collective Market Share Approximately 45% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, Japan, South Korea, India |
| Future outlook |
|
What are the growth opportunities in this market?
Peptide Synthesis Reagents Market Trends
Four strengthening trends—advanced synthesis integration, commercial-scale manufacturing, biomarker-driven product commercialization, precision medicine adoption, and digital transformation—are reshaping the peptide synthesis reagents market by driving innovations in R&D investments, specification updates, and procurement strategies.
- Given that commercial-scale peptide synthesis systems are being incorporated into pharmaceutical development and biotechnology applications with proven efficacy records at established facilities, ongoing innovation continues to be driven forward. Commercial facilities for peptide manufacturing as demonstrated in recent industry developments emphasize the growing need for scalability in therapeutic peptide engineering. In the short to medium term, industrial processing and commercial reagent production will be designed around scaling up continuous manufacturing systems with efficiency improvements of up to 35-45% cost reductions at the facility level, leading to capacity expansions among major producers that are vigorously fueling demand for advanced peptide synthesis systems and compatible processing technologies for developing applications in pharmaceutical and precision medicine production.
- In the long term, steady scale-up commercialization is being undertaken for peptide synthesis systems and specialized bioactive variants designed for improved therapeutic efficacy and processing efficiency. These innovations enable commercial-scale manufacturing techniques in personalized processing, conventional synthesis production, and hybrid approaches, improving facility performance. Patent activity is advancing in parallel, indicating considerable R&D investment. However, there is increasing demand in high-performance clinical applications where personalized and targeted features are prioritized over cost, even though such applications remain approximately 3-5 times more expensive than conventional systems. Accordingly, market production development will focus on specialized synthesis systems relevant under recognized regulatory and precision medicine frameworks.
- Peptide synthesis processes are undergoing transformation aligned with personalized medicine and precision therapeutic targets. As manufacturers seek to optimize therapeutic efficacy outcomes, they are implementing targeted bioactive sourcing, closed-loop synthesis systems, and pharmaceutical-integrated processing. The application of precision medicine principles to commercial-scale production will bring substantial benefits in the medium term. These immediate improvements enhance vendor competitiveness by achieving therapeutic improvements of 45-65% and enhancing drug efficacy, while future synthesis certifications such as clinical validation standards and pharmaceutical assessments will develop into procurement differentiators.
- Manufacturing of peptide synthesis reagents is undergoing digital transformation, enhancing precision and consistency. Manufacturers streamline monitoring of bioactive operations through the Internet of Things (IoT), Artificial Intelligence (AI)-guided quality control, and real-time synthesis analytics to optimize formulation performance and therapeutic outcomes. Digital workflows can offer precision improvements of 25-35%, especially within commercial-scale peptide manufacturing environments. Therefore, the market is favoring suppliers capable of offering reproducibility, traceability, and fast-track processing backed by substantial evidence-based documentation and remote monitoring capabilities, driving improved digital maturity in peptide synthesis reagent production.
Peptide Synthesis Reagents Market Analysis
Learn more about the key segments shaping this market
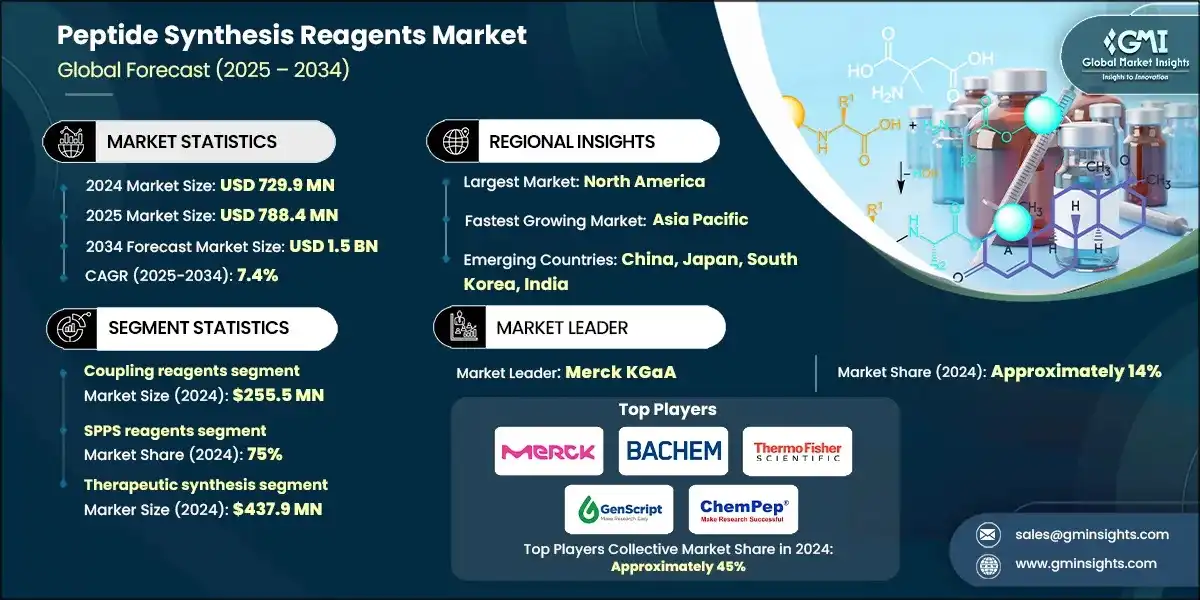
Based on reagent product type, the coupling reagents segment was valued at USD 255.5 million in 2024 and is anticipated to expand at a CAGR of 7.6% during 2025 to 2034, holding a market share of 35% in 2024.
- Standalone carbodiimide-based coupling innovations, broad versatility in peptide synthesis applications, and established efficacy standards provide a strong foothold for coupling reagent systems within the peptide synthesis reagents segment. Its peptide bond formation capability is scalable, along with optimizing the activation profile of coupling formulations, which is the most accepted technology for commercial peptide synthesis and thereby creates an ever-steady demand within the research, pilot, and commercial production ambience. Processing of large-scale manufacturing receives an additional advantage with the integration of advanced uronium and phosphonium systems with examples of HATU and PyBOP workhorse systems.
- Protecting group reagents, which oversee the selective protection processes in pharmaceutical facilities to enhance synthesis efficiency and guarantee regulatory compliance, are ranked second with 25% market share within reagent product types. Superior synthesis performance and real-time amino acid protection are offered by suppliers, who have popularized their use in commercial-scale peptide synthesis production and precision pharmaceutical applications.
- Next-generation green coupling reagents is the segment that maintains steady growth in the reagent product type category with 9% CAGR due to this being the game changer in developing environmentally-targeted products with complex bioactive architecture that is best suited for sustainable applications. Their premium positioning because of the challenging technology development and the exceptional synthesis differentiation that these systems provide in sustainable peptide production, eco-friendly alternatives, and specialty therapeutic formulations against traditional coupling methods. The peptide synthesis reagent products are increasingly going forward to differentiate between volume-using traditional coupling equipment on one side, and value-adding personalized technologies on the other, thus the way in which the competition among suppliers evolve through precision level, therapeutic efficiency, and specialized pharmaceutical integration.
Learn more about the key segments shaping this market
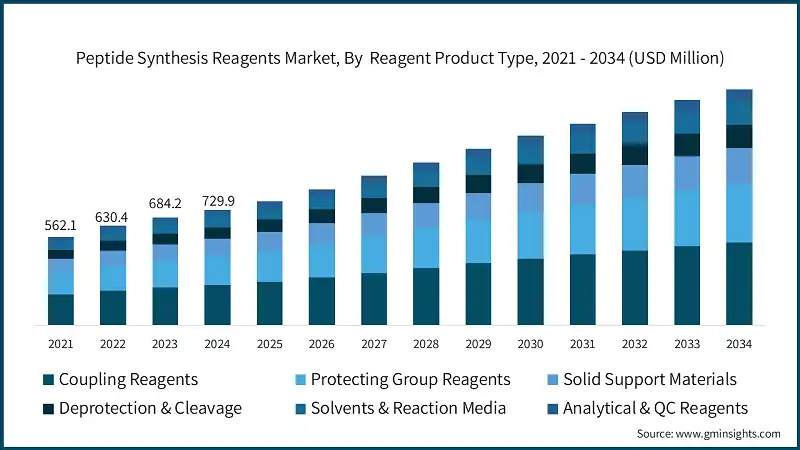
Based on synthesis method, the peptide synthesis reagents market from SPPS reagents segment was valued at USD 547.4 million in 2024 and is anticipated to expand at a CAGR of 7.5% during 2025 to 2034, holding a market share of 75% in 2024.
- Standalone Fmoc SPPS innovations, broad versatility in automated synthesis applications, and established efficacy standards provide a strong foothold for SPPS reagent systems within the peptide synthesis reagents segment. Its solid-phase synthesis capability is scalable, along with optimizing the coupling profile of Fmoc formulations, which is the most accepted technology for commercial peptide production and thereby creates an ever-steady demand within the research, pilot, and commercial production ambience. Processing of large-scale manufacturing receives an additional advantage with the integration of advanced microwave SPPS systems with examples of automated compatible and high-throughput workhorse systems.
- LPPS reagents, which used in the solution-phase synthesis processes in pharmaceutical facilities to enhance peptide production flexibility and guarantee regulatory compliance, are ranked second with 20% market share within synthesis method types. Superior synthesis performance and real-time fragment condensation are offered by suppliers, who have popularized their use in commercial-scale liquid-phase synthesis production and precision pharmaceutical applications.
- Hybrid and advanced synthesis represents the fastest-growing segment in the synthesis method category, projected to register a steady CAGR of 7.1% from 2025 to 2034. This growth is driven by its pivotal role in enabling technologically advanced products with complex synthetic architectures tailored for specialized applications. Its premium positioning arises from the sophisticated technology development and superior synthesis differentiation these systems deliver in continuous flow chemistry, convergent synthesis, and specialty enzymatic formulations compared to traditional solid-phase methods.
Based on application, the peptide synthesis reagents market from therapeutic synthesis segment was valued at USD 437.9 million in 2024 and is anticipated to expand at a CAGR of 7.2% during 2025 to 2034, holding a market share of 60% in 2024.
- Standalone GMP-grade manufacturing innovations, broad versatility in pharmaceutical applications, and established regulatory standards provide a strong foothold for therapeutic synthesis systems within the peptide synthesis reagents segment. Its clinical-grade synthesis capability is scalable, along with optimizing the purity profile of therapeutic formulations, which is the most accepted technology for commercial drug development and thereby creates an ever-steady demand within the research, pilot, and commercial production ambience. Processing of large-scale manufacturing receives an additional advantage with the integration of advanced long peptide specialized systems with examples of modified peptide reagents and clinical-grade workhorse systems.
- R&D applications, which manage research and development processes in pharmaceutical facilities to enhance drug discovery efficiency and ensure innovation compliance, hold the second-largest market share at 30% in 2024 among application types. Suppliers offer superior research performance and real-time high-throughput screening capabilities, driving their adoption in commercial-scale library synthesis and precision pharmaceutical applications.
- Diagnostic & analytical is the segment that maintains steady growth in the application category with 7.4% CAGR from 2025 to 2034 due to this being the game changer in developing diagnostically-targeted products with complex biomarker architecture that is best suited for analytical applications. Their premium is placed because of the challenging technology development and the exceptional synthesis differentiation that these systems provide in biomarker peptide synthesis, radiopharmaceutical precursors, and specialty immunoassay formulations against traditional therapeutic methods.
Looking for region specific data?
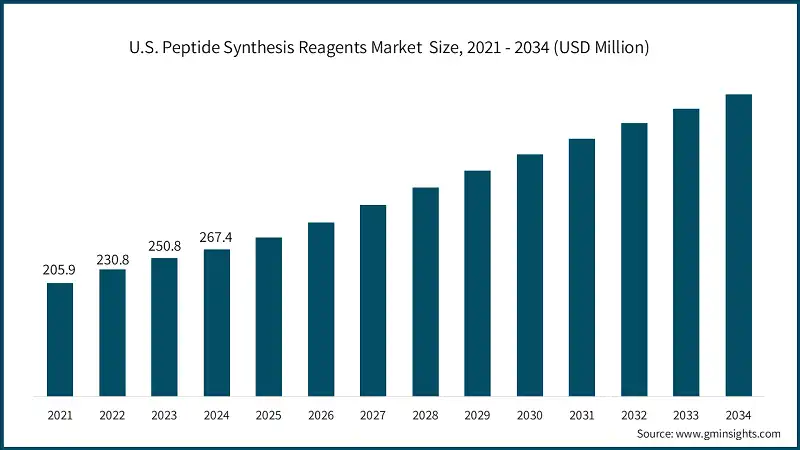
- In the North America peptide synthesis reagents market, the U.S. is the dominant country, valued at USD 267.4 million in 2024, holding the majority of the market share due to strong government support for pharmaceutical research initiatives, advanced peptide synthesis research infrastructure, and the presence of key industry players. The North American market revolves around requirements for pharmaceutical safety standards, FDA regulations, and innovations in peptide synthesis technology. Supplier requirements and procurement standards have been informed and developed via drug development frameworks as well as clinical validation standards. Personalized medicine as well as precision therapeutic initiatives continue to sustain downstream demand for advanced peptide synthesis reagent systems.
- Government-funded research programs are actively advancing peptide synthesis technologies, personalized medicine systems, and next-generation therapeutic peptide processing, supported by initiatives from the NIH and national health agencies. Key participants include Merck KGaA, known for its advanced Fmoc systems and synthesis expertise; Thermo Fisher Scientific, specializing in reagents and analytical instruments; and several peptide synthesis firms focused on therapeutic development platforms. National research funding bodies in countries such as the United States and Canada continue to support projects centered on precision medicine, personalized therapeutic performance, and pharmaceutical technologies integrated with peptide synthesis innovations.
The Europe peptide synthesis reagents market was valued at USD 241 million in 2024, and it is anticipated to expand at 7.1% CAGR during 2025-2034, holding a market share of 33% in 2024.
- In Europe, Germany represents the largest market for peptide synthesis reagent products, valued at USD 86.6 million in 2024, and is projected to continue providing leadership in technological aspects for the region. Germany boasts the fastest pace of innovation adoption owing to established peptide synthesis research infrastructure, precision medicine industry, and significant investments towards personalized therapeutic applications. Substantial government funding, along with regulatory support directed at advanced personalized synthesis systems and new companies developing reagent technologies, positions Germany as a heavyweight consumer while a technological leader in the global peptide synthesis reagents market.
- The market for peptide synthesis reagent products, in terms of both share and technological advancement, is driven by the growing adoption of precision medicine infrastructure and a strong regulatory framework. High utilization of peptide synthesis technologies and increasing integration of personalized therapeutic systems are observed across precision medicine activities, commercial manufacturing, and research applications. Regulatory efforts aimed at ensuring clarity and accelerating approval processes are fast-tracking the commercialization of peptide synthesis materials and next-generation personalized systems. Additionally, supply chains are evolving to support scalable manufacturing, integrated production systems, personalized controls, and modular designs—enhancing both commercial sustainability and R&D progress.
The Asia Pacific peptide synthesis reagents market was valued at USD 124.2 million in 2024 and dominated the fastest-growing segment with a CAGR of 7.5% during 2025-2034, holding a market share of 17% in 2024.
- Furthermore, the Asia Pacific market for peptide synthesis reagent products has been characterized by the high manufacturing capabilities in the region, sustained investment by governments, and developing precision medicine infrastructures in the countries participating in the region. China is the dominant country in the region, valued at USD 49.8 million in 2024. China's precision therapeutic programs, advanced manufacturing policies, and other national initiatives promoting the establishment of peptide synthesis production systems and personalized medicine would eventually increase the demand for high-efficiency therapeutic materials and peptide synthesis reagent systems. Regulatory frameworks formalizing personalized medicine and technology validation will be greatly beneficial for commercial scaling in both Japan and South Korea.
- Government support in India and Australia is increasing further advancements in technology adoption for modern personalized manufacturing systems and precision processing. The Asia Pacific market is also related to the traditional pharmaceutical modernization initiatives with personalized alternatives, while those in China are equipped with manufacturing capabilities and academic consortia for innovating peptide synthesis production systems and safety studies for therapeutic materials.
The Latin America peptide synthesis reagents market was valued at USD 29.2 million in 2024 and accounted for 4% market share in 2024.
- Research and commercial activities across Latin America, particularly in Brazil, Mexico, and Argentina, are being supported by expanding personalized medicine modernization programs and newly funded precision therapeutic initiatives. Brazil leads the regional peptide synthesis reagents market, valued at USD 14.9 million in 2024, with steady growth projected over the analysis period. The market opportunity in the region is driven by rising interest in peptide synthesis production technologies and the continuous development of pharmaceutical capabilities. Brazil’s strong chemical industry and expanding research base provide a solid foundation for the broader adoption of advanced peptide synthesis reagent systems across Latin America.
- The well-developed chemical operations in Brazil, along with their expanding pharmaceutical programs, make Brazil the leading country in that region. The commercial sector strongly benefits from support from the government and international partnerships, as well as an increasing focus on personalized medicine applications, which results in a strong ongoing demand for advanced peptide synthesis reagent systems in commercial manufacturing and aspiring precision therapeutic applications.
The Middle East & Africa peptide synthesis reagents market was valued at USD 14.3 million in 2024 and is anticipated to grow at a CAGR of 6.3% during 2025 to 2034, holding a market share of 2% in 2024.
- The Middle East and Africa are the main and significant places for the great likelihoods of growth dominated by the establishment of precision medicine mechanisms, along with investments in developing peptide synthesis technologies, and the interest in sophisticated personalized therapeutic applications. UAE continues to hold a dominant position in the Middle East & Africa peptide synthesis reagents market, valued at USD 3.9 million in 2024 and projected to reach USD 8 million by 2034 at a CAGR of 7.3%, due to its major growth potential. Investment in pharmaceutical and technology infrastructure creates parameters that open opportunities for peptide synthesis applications in many stages of commercial development and research activities in UAE, Saudi Arabia, and South Africa.
- UAE has become a regional leader due to heavy investments in precision medicine and peptide synthesis research under the UAE Vision 2071 initiative. With the establishment of advanced research facilities and the increase in personalized therapeutic programs, UAE is emerging as a critical market as regard to commercialization of modern peptide synthesis materials and new precision medicine applications, all aimed at the production of personalized therapeutic alternatives.
Peptide Synthesis Reagents Market Share
In 2024, the global peptide synthesis reagents industry was led by a diverse group of chemical manufacturers, life science suppliers, and specialty biotechnology providers, where the top seven companies collectively accounted for approximately 42% of the market share in 2024, including Merck KGaA, Bachem AG, Thermo Fisher Scientific, GenScript Biotech, ChemPep Inc., AAPPTec/Advanced ChemTech, and Iris Biotech GmbH. These companies hold strong market positions due to their advanced peptide synthesis formulations, large-scale manufacturing technologies, precision therapeutic reagents, and strategic partnerships with pharmaceutical organizations, enabling efficient peptide synthesis reagent manufacturing, scaling, and therapeutic peptide optimization.
- Merck KGaA specializes in the design and manufacture of advanced Fmoc-based coupling systems and protecting group solutions for pharmaceutical, biotechnology, and precision medicine industries. Its product range supports commercial to clinical-level operations, emphasizing biocompatibility, scalability, and process control for therapeutic peptide applications, synthesis materials, and personalized medicine solutions. The company's flagship Novabiochem peptide synthesis reagents represent the industry standard for Fmoc-based peptide supplementation with advanced coupling technology integration across over 40 manufacturing plants globally.
- Bachem AG is a global peptide synthesis reagent manufacturer and supplier offering innovative Fmoc-based, Boc-enhanced, and comprehensive therapeutic synthesis solutions for the pharmaceutical and biotechnology industry. The company's products include advanced peptide synthesis formulations, coupling reagent systems, and therapeutic development technologies that support precision peptide manufacturing and pharmaceutical facility optimization. Bachem focuses on scientific research, GMP validation, and technological innovation to accelerate the development and production of peptide synthesis applications. Their market-leading Fmoc amino acid derivatives maintain significant market presence in pharmaceutical facilities with FDA-approved formulations and cGMP manufacturing technology integration. Key products include
- Thermo Fisher Scientific is a leading peptide synthesis reagent company that develops and provides comprehensive coupling formulations, precision therapeutic synthesis, and specialized pharmaceutical solutions for research and commercial applications. Its product range includes advanced Fmoc systems, targeted synthesis platforms, and precision medicine technologies. Thermo Fisher supports commercial, pharmaceutical, and biotechnology markets globally by improving therapeutic development, peptide manufacturing, and personalized medicine enhancement in synthesis processes. Their premium Fmoc amino acid catalog represents validated innovation with proven efficacy in SPPS applications, automated synthesis, and clinical-grade peptide production through Pierce Biotechnology partnership technology. Key products include.
Peptide Synthesis Reagents Market Companies
The major players operating in peptide synthesis reagents industry include:
- Merck KGaA
- Bachem AG
- Thermo Fisher Scientific
- GenScript Biotech
- ChemPep Inc.
- AAPPTec / Advanced ChemTech
- CSBio Company
- Iris Biotech GmbH
- GL Biochem (Shanghai) Ltd
- Peptides International (Biosynth/vivitide)
- Biosynth (vivitide, Pepscan, CRB, Pepceuticals)
- AmbioPharm Inc.
- Creative Peptides
- Peptide Institute, Inc.
- CEM Corporation
Peptide Synthesis Reagents Industry News
- In August 2025, Cambrex announced that its subsidiary, Snapdragon Chemistry, has expanded its API manufacturing facility in Waltham, Massachusetts, to enhance peptide therapy development and production. The expansion adds a new GMP suite with an ISO-7 cleanroom, preparative HPLC, lyophilization, and cold storage, increasing the site’s footprint by 20%. The upgraded facility enables Snapdragon to support peptide projects from development through GMP manufacturing using solid-phase (SPPS), liquid-phase (LPPS), or hybrid synthesis approaches.
- In February 2025, Granules India announced the signing of an acquisition agreement to purchase Senn Chemicals AG, a Switzerland-based CDMO specializing in peptide development and manufacturing. The deal, expected to close in the first half of 2025 pending customary conditions, marks Granules’ entry into the fast-growing peptide therapeutics segment and strengthens its CDMO business. Senn brings expertise in Liquid-Phase and Solid-Phase Peptide Synthesis (LPPS and SPPS), a cGMP-certified facility approved by Swissmedic, and established relationships with global pharma and cosmetics clients.
The peptide synthesis reagents market research report includes in-depth coverage of the industry with estimates & forecast in terms of revenue (USD Million) & (Kilo Tons) from 2021 to 2034, for the following segments:
Market, By Reagent Product Type
- Coupling reagents
- Carbodiimide-based
- Phosphonium-based
- Uronium-based
- Immonium-based
- Next-gen green
- Protecting group reagents
- Fmoc reagents & derivatives
- Boc reagents & derivatives
- Side-chain protecting
- Orthogonal systems
- Solid support materials
- Polystyrene-based resins
- Peg-based & chemmatrix
- Specialty resins
- Biodegradable materials
- Deprotection & cleavage
- Base systems
- Acid cleavage reagents
- Scavenger systems
- Cleavage cocktail formulations
- Solvents & reaction media
- Traditional solvents
- Green solvent alternatives
- Ionic liquids & deep eutectic
- Aqueous & bio-compatible
- Analytical & QC reagents
- HPLC Standards & reference
- Kaiser test & ninhydrin
- Mass spectrometry standards
- Purity assessment reagents
Market, By Synthesis Method
- SPPS Reagents
- Fmoc SPPS systems
- Boc SPPS systems
- Microwave SPPS
- Automated compatible
- LPPS reagents
- Solution-phase systems
- Fragment condensation
- Purification-compatible
- Hybrid & advanced
- Continuous flow chemistry
- Convergent synthesis
- Enzymatic ligation
- Click chemistry systems
Market, By Application
- Therapeutic synthesis
- GMP-grade manufacturing
- Clinical-grade (phase I-III)
- Long peptide specialized
- Modified peptide reagents
- R&D applications
- High-throughput screening
- Library synthesis
- Proof-of-concept & lead Opt
- Academic research grade
- Diagnostic & analytical
- Biomarker peptide synthesis
- Radiopharmaceutical precursor
- Immunoassay standards
- Specialty applications
- Cosmetic peptide synthesis
- Food & nutraceutical grade
- Agricultural peptide synthesis
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- MEA
- UAE
- Saudi Arabia
- South Africa
- Rest of Middle East and Africa
Frequently Asked Question(FAQ) :
Who are the key players in the peptide synthesis reagents market?
Key players include Merck KGaA, Bachem AG, Thermo Fisher Scientific, GenScript Biotech, ChemPep Inc., AAPPTec / Advanced ChemTech, and Iris Biotech GmbH. These companies focus on advanced Fmoc-based systems, scalable synthesis technologies, and personalized peptide manufacturing solutions for pharmaceutical and biotechnology industries.
What are the upcoming trends in the peptide synthesis reagents industry?
Key trends include integration of AI- and IoT-enabled synthesis monitoring, commercial-scale manufacturing automation, and the adoption of green and bio-based coupling reagents for sustainable peptide production.
Which region leads the peptide synthesis reagents market?
The U.S. peptide synthesis reagents industry was valued at USD 267.4 million in 2024, holding the majority share in North America. Growth is driven by strong government support for peptide R&D, NIH-funded programs, and established pharmaceutical infrastructure enabling advanced therapeutic development.
What is the growth outlook for the therapeutic synthesis application segment from 2025 to 2034?
The therapeutic synthesis segment is projected to grow at a 7.2% CAGR through 2034.
What was the valuation of the SPPS reagents segment in 2024?
The solid-phase peptide synthesis (SPPS) reagents segment was valued at USD 547.4 million in 2024, holding a 75% share, driven by automation compatibility and efficiency in large-scale peptide production.
How much revenue did the coupling reagents segment generate in 2024?
The coupling reagents segment generated USD 255.5 million in 2024, leading the market with a 35% share.
What is the projected value of the peptide synthesis reagents market by 2034?
The peptide synthesis reagents market is expected to reach USD 1.5 billion by 2034, driven by the expansion of personalized medicine, large-scale therapeutic peptide manufacturing, and adoption of automated synthesis systems.
What is the market size of the peptide synthesis reagents industry in 2024?
The market size was USD 729.9 million in 2024, with a CAGR of 7.4% expected through 2034 driven by rising demand for peptide therapeutics, biopharmaceutical innovation, and advancements in precision medicine.
What is the current peptide synthesis reagents market size in 2025?
The market size is projected to reach USD 788.4 million in 2025, supported by growing R&D investments in peptide drug discovery and commercialization of solid-phase synthesis technologies.
Peptide Synthesis Reagents Market Scope
Related Reports


