Summary
Table of Content

Minimally Invasive Spine Surgery Devices Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Minimally Invasive Spine Surgery Devices Market Size
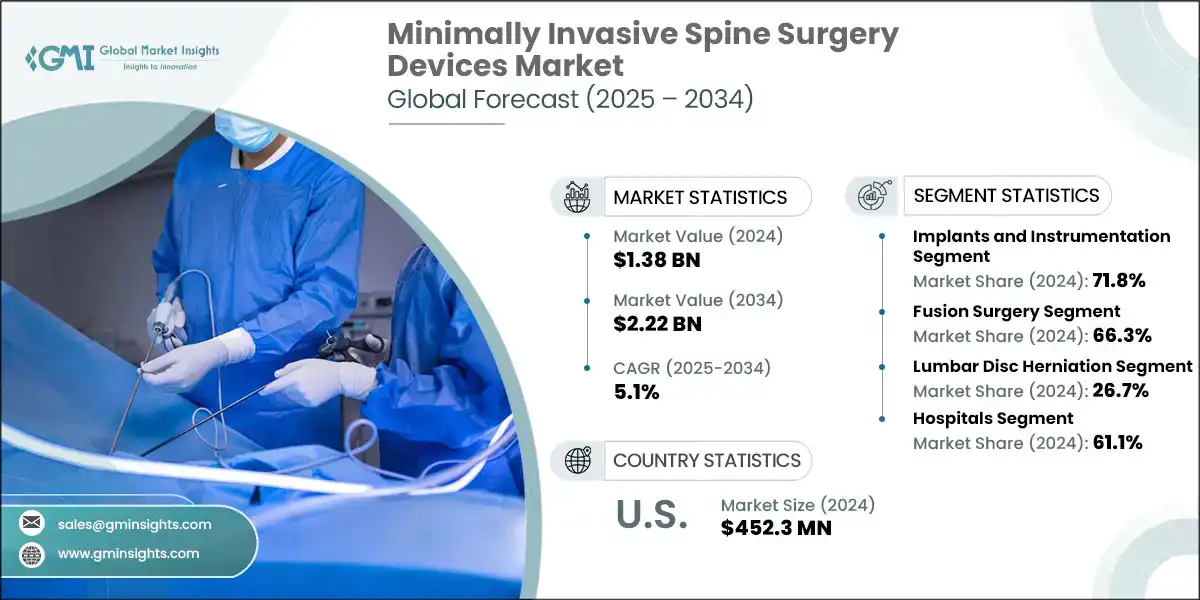
The global minimally invasive spine surgery devices market was estimated at USD 1.38 billion in 2024. The market is expected to grow from USD 1.42 billion in 2025 to USD 2.22 billion in 2034, growing at a CAGR of 5.1%. The high demand for minimally invasive spine surgery devices is increasing due to the presence of smaller incisions along with reduced hospital stays as well as faster recovery times. These methods lessen the pain. Since they also reduce scars, patients often prefer them. Developments within both medical imaging and navigation technologies also support precision, and in turn, this makes the procedures safer along with more common adoption.
To get key market trends
Spinal procedures use minimally invasive spine surgery devices, as these tools limit tissue and muscle damage. These devices allow for faster healing. They also lead to less postoperative pain for the patients. Since the demand for these procedures is rising, the global market is also expanding. Medtronic, DePuy Synthes, NuVasive, Stryker, Zimmer Biomet, and others are a few of the key players in the market.
The market reached USD 1.27 billion in 2021 and USD 1.33 billion in 2023, registering a CAGR of 2.4% during this period. This growth is driven by the rising cases of spinal disorders, increasing preference for procedures that are less invasive, and the aging global population. These techniques allow for faster recovery and reduce complications for both patients and surgeons. Furthermore, surgical tools and imaging technologies continuously advance, supporting broader adoption throughout hospitals and outpatient surgical centers.
Aging demographics and the increasing incidence of spinal conditions drive growth in the minimally invasive spine surgery (MISS) devices market. Moreover, patients and healthcare providers increasingly realize the benefits of minimally invasive procedures as a key driver. These include reduced postoperative pain, lowered risks of infection, shorter hospital stays, and faster returns to daily activities. Healthcare systems prioritize procedures offering better outcomes with lower costs as they shift to value-based care, thus increasing MISS technique adoption.
The rise of outpatient or ambulatory surgical centers has created a strong demand for compact, efficient devices suited to minimally invasive procedures. These centers' capabilities align well with MISS techniques, supporting market expansion beyond customary hospital settings. Surgeons can access more training and skill development programs, which is a positive factor. These programs are increasingly available now. More orthopedic and neurosurgeons become skilled in MISS techniques as adoption spreads widely across geographies and institutions.
Revolutionary technology matters just as much. As robotics, 3D navigation, real-time imaging, and augmented reality have been integrated into spine surgery, surgical accuracy improves, and the learning curve lessens, encouraging facilities to invest in these systems. The demand for less invasive treatments further increases along with the growing burden from obesity and sedentary lifestyles that often contribute to spinal issues. In developed countries, reimbursement improvements and expanding healthcare access in emerging economies, among other factors, add to the momentum, helping the global market maintain steady growth over the coming years.
Minimally invasive spine surgery devices are specialized tools and instruments used to perform spinal procedures through small incisions. These devices help reduce muscle damage, blood loss, and recovery time compared to traditional open surgeries. They include spinal implants, handheld and powered surgical instruments, and imaging systems designed to improve precision, safety, and patient outcomes.
Minimally Invasive Spine Surgery Devices Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 1.38 Billion |
| Forecast Period 2025 – 2034 CAGR | 5.1% |
| Market Size in 2034 | USD 2.22 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising prevalence of spine disorders | Increasing use of wearable injectors for managing chronic diseases, improving adherence, and reducing hospital visits. |
| Growing geriatric population | Growing adoption of wearable injectors for at-home treatment is easing pressure on clinical settings. |
| Increased adoption of outpatient spine surgeries | Wearable injectors support convenient, patient-friendly subcutaneous delivery of biologics that require specialized administration. |
| Technological advancements in navigation and robotics | Patients favor wearable injectors for their discreet, needle-free-like experience and ease of use, enhancing adherence and satisfaction. |
| Pitfalls & Challenges | Impact |
| High cost of advanced MISS equipment | High costs limit adoption in price-sensitive markets and smaller healthcare settings. |
| Lack of skilled surgeons in developing regions | Design limitations affect usability, reliability, and safety, lowering confidence and increasing failure rates. |
| Opportunities: | Impact |
| Integration with AI and augmented reality | Fuels innovation in on-body systems, positioning wearable injectors as the go-to for chronic and specialty biologics. |
| Development of cost-effective MISS solutions | Enables integration with digital platforms for real-time monitoring and proactive care. |
| Market Leaders (2024) | |
| Market Leaders |
31.2% Market share |
| Top Players |
Collective market share in 2024 is 78.3% |
| Competitive Edge |
|
| Regional Insights | |
| Largest market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | India, Brazil, Mexico, Indonesia, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Minimally Invasive Spine Surgery Devices Market Trends
- The market for MISS devices is influenced by the convergence of macro-level and micro-level trends. On a macro scale, the aging global population and the increasing prevalence of spinal disorders have significantly driven the demand for MISS solutions.
- Healthcare payers are emphasizing value-based care, prioritizing shorter hospital stays, which encourages providers to adopt minimally invasive options. These options not only reduce overall costs but also enhance patient throughput.
- The industry is leveraging platforms that incorporate haptic feedback, automated trajectory planning, and AI to enhance decision-making during surgeries. Robotic systems assist surgeons in achieving sub-millimeter accuracy for pedicle screw placement, improving outcomes and reducing complications in spinal fusion procedures. Image-guided robotic surgeries provide real-time anatomical guidance, enhancing precision in tissue-sparing MISS procedures by utilizing intraoperative CT, MRI, and augmented reality overlays.
- At the micro level, manufacturers are utilizing 3D printing and advanced biomaterials such as PEEK, titanium alloys, and injectable porous foam to develop personalized spinal implants tailored to individual anatomies. These implants improve osteointegration and reduce complication rates, while also supporting customized surgical planning.
- Additionally, regenerative biologics, including stem cells and growth factor-enhanced grafts, are emerging to support fusion and repair discs in conjunction with MISS devices.
- The regulatory and reimbursement landscape continues to evolve. Companies are reevaluating supply chains and forming domestic manufacturing partnerships in regions like North America and the EU to align with new import tariff regimes and medical device regulations. Outcome-based contracting and bundled procedure pricing are gaining traction, helping to distribute financial risk and justify investments in advanced MISS equipment.
- The industry is also adopting smart manufacturing methods, such as additive production, traceable cradle-to-grave supply chains, and on-demand sterile vending solutions, to streamline inventory management and deliver patient-specific devices efficiently.
- Innovative tools, including guidance devices, are facilitating the growth of MISS in outpatient clinics. Additionally, surgeon education programs are enhancing skill adoption in hospitals across emerging markets. Telemedicine and remote monitoring are expanding, integrating wearables and digital recovery tracking to improve patient outcomes.
- These macro and micro trends, along with advancements in technology, regulatory changes, and innovations in product manufacturing, are driving the MISS devices market toward rapid growth, emphasizing personalized, precision-based care, cost-effective delivery, and scalable innovation.
Minimally Invasive Spine Surgery Devices Market Analysis
Learn more about the key segments shaping this market
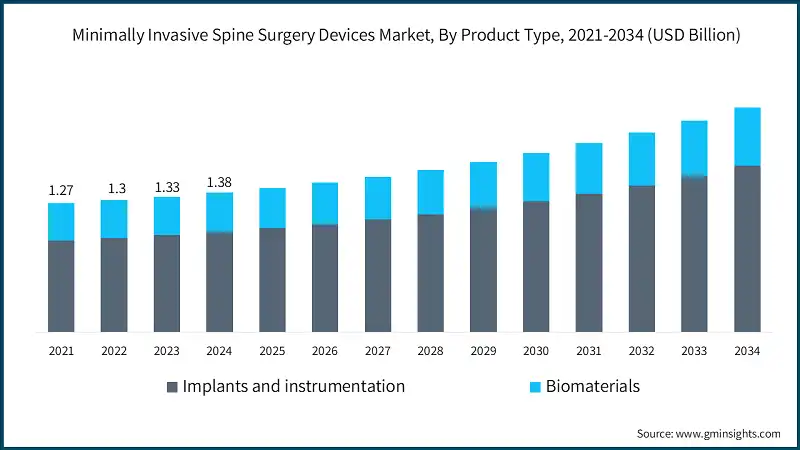
The global market reached from USD 1.27 billion in 2021 to USD 1.33 billion in 2023, driven by the rising demand for shorter hospital stays and faster recovery times associated with minimally invasive spine procedures.
Based on the product type, the market is bifurcated into implants and instrumentation, and biomaterials. The implants and instrumentation segment accounted for 71.8% of the market in 2024 due to the increasing adoption of advanced fixation systems and spinal implants for improved surgical precision and patient outcomes. The segment is expected to exceed USD 1.6 billion by 2034, growing at a CAGR of 5.4% during the forecast period. On the other hand, biomaterials segment is expected to grow with a CAGR of 4.2%. The growth of this segment can be attributed to increasing demand for advanced bone graft substitutes that enhance spinal fusion and healing outcomes.
- The implants and instrumentation segment leads the market due to the growing adoption of navigation-assisted tools, interbody cages, and advanced spinal fixation systems. These devices are increasingly favored by surgeons as they enhance surgical precision, support faster patient recovery, provide better stability, and reduce intraoperative risks. The shift toward image-guided and robotic-assisted surgeries further drives the adoption of these solutions.
- Additionally, advancements in implant materials, such as titanium and carbon fiber composites, have improved biocompatibility and durability. These innovations make such solutions ideal for complex spinal procedures, contributing to the segment's sustained market growth.
- The biomaterials segment is experiencing growth due to the rising demand for advanced bone graft substitutes, which accelerate and enhance spinal fusion procedures. Surgeons increasingly prefer these materials as they promote better healing, reduce complications, and eliminate the need for autografting. Technological advancements in synthetic and biologic graft materials are further driving their adoption across minimally invasive spine procedures.
Based on the application, the minimally invasive spine surgery devices market is segmented into fusion surgery, and non-fusion surgery. The fusion surgery segment accounted for the highest market share of 66.3% in 2024, driven by the increasing prevalence of degenerative disc diseases and demand for spinal stabilization procedures.
- The fusion surgery segment leads the market, driven by the rising prevalence of degenerative disc diseases, spinal stenosis, and spondylolisthesis. These conditions often necessitate spinal stabilization, making fusion procedures the preferred treatment option. Minimally invasive fusion techniques are increasingly favored by both patients and surgeons due to reduced trauma and faster recovery times.
- Technological advancements in fusion devices, such as enhanced interbody cages and bone graft materials, have improved surgical outcomes, further driving their adoption in both outpatient and hospital settings.
- The non-fusion surgery segment, accounting for 33.7% of the market share, is projected to grow at a CAGR of 4.4% during the forecast period. The demand for motion-preserving procedures is increasing as patients seek alternatives to spinal fusion to minimize long-term complications. Innovations such as artificial discs and dynamic stabilization devices are gaining traction, offering improved mobility and enhanced quality of life post-surgery.
Based on indication, the minimally invasive spine surgery devices market is segmented into lumbar disc herniation, spinal stenosis, degenerative spinal disease, cervical disc disorders, thoracic disc herniation, and other indications. The lumbar disc herniation segment accounted for the highest market share of 26.7% in 2024, driven by its high global prevalence and the growing preference for minimally invasive discectomy procedures.
- The lumbar disc herniation segment leads the market due to its high global prevalence, particularly among adults with sedentary or physically demanding jobs. Consequently, minimally invasive discectomy procedures have gained popularity as they offer faster recovery times and reduced postoperative discomfort. These surgeries minimize tissue damage, shorten hospital stays, and enable quicker return to normal activities. Technological advancements in navigation and imaging have further enhanced surgical precision, solidifying the preference for minimally invasive approaches in treating lumbar disc herniation across various healthcare settings.
- The spinal stenosis segment, the second largest, accounted for a market share of 21.6% in 2024. Increased awareness and improved diagnosis rates have driven demand for minimally invasive treatment options. Patients with spinal stenosis often prefer procedures that provide symptom relief with minimal disruption, making minimally invasive devices an attractive choice.
- Technological advancements in imaging, surgical navigation, and interbody fusion techniques have enhanced treatment precision, supporting broader adoption. Additionally, reimbursement support and reduced postoperative risks have made these procedures more accessible and widely preferred.
- The degenerative spinal disease segment, while smaller with a market share of 19.6%, is projected to grow at a CAGR of 5%. This growth is attributed to the rising aging population, as spinal degeneration is more common among older adults. Sedentary lifestyles and obesity are also contributing to earlier onset of these conditions. Increased awareness, improved diagnosis, and a growing preference for minimally invasive treatments are driving the adoption of advanced surgical devices in this segment.
Learn more about the key segments shaping this market
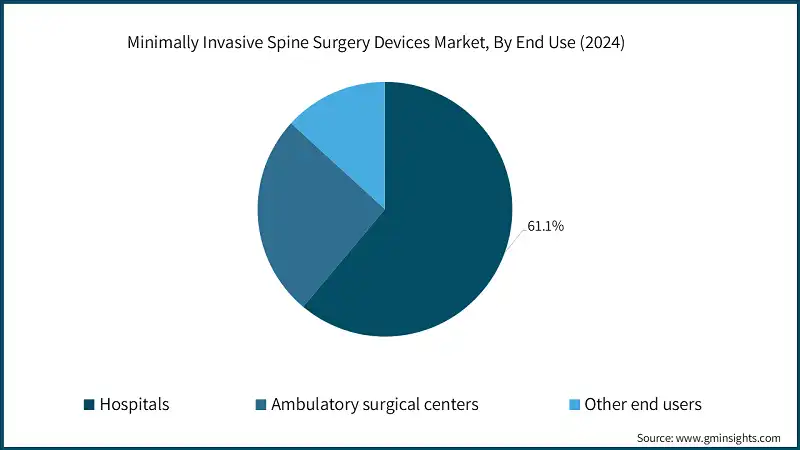
Based on the end use, the minimally invasive spine surgery devices market is segmented into hospitals, ambulatory surgical centers, and other end users. The hospitals segment accounted for the highest market share of 61.1% in 2024, driven by the availability of advanced surgical infrastructure and skilled professionals for complex minimally invasive spine procedures.
- The hospital segment accounts for the largest share of the market, contributing significantly to the overall market value. This dominance is attributed to hospitals' access to advanced surgical infrastructure, including high-end imaging systems and robotic-assisted tools with real-time navigation technologies. These facilities attract skilled surgeons capable of performing complex spine procedures with high precision.
- Hospitals also function as referral centers for severe spinal conditions, leading to higher procedural volumes. Their comprehensive post-operative care, integrated services, and better reimbursement support make them the preferred choice for both patients and healthcare providers.
- The ambulatory surgical centers (ASCs) segment, while holding a smaller market share of 25.7%, is projected to grow at a CAGR of 5.7% during the forecast period. This growth is driven by the cost-efficiency, faster patient turnover, and shorter procedure times offered by ASCs. Minimally invasive spine surgeries, which require less recovery time, align well with the capabilities of ASCs. Additionally, increasing patient preference for outpatient care, advancements in surgical tools, and favourable reimbursement policies are further propelling the growth of this segment.
Looking for region specific data?
North America dominated the global minimally invasive spine surgery devices market with the highest market share of 35.3% in 2024.
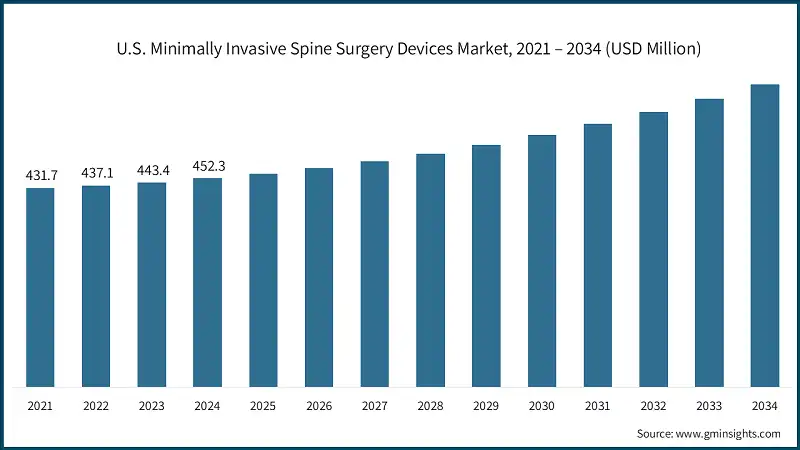
- The U.S. market was valued at USD 431.7 million and USD 437.1 million in 2021 and 2022, respectively. In 2024 the market size reached USD 452.3 million from USD 443.4 million in 2023. This growth is driven by the rising prevalence of degenerative spinal disorders and increasing adoption of advanced surgical technologies.
- North America leads the market due to several contributing factors. The region's well-established healthcare infrastructure facilitates the rapid adoption of advanced surgical techniques and devices. Additionally, high awareness among patients and healthcare providers regarding the benefits of minimally invasive procedures drives demand. The presence of key medical device manufacturers in the region further promotes continuous innovation and product availability.
- Favorable reimbursement policies and insurance coverage for advanced spinal procedures encourage healthcare facilities to adopt these technologies. Outpatient surgical centers, known for their efficiency and faster patient turnover, increasingly prefer minimally invasive techniques, further boosting investments and market growth.
- In the U.S., market growth is primarily driven by the rising prevalence of spinal disorders, attributed to aging populations, obesity, and sedentary lifestyles. This has led to strong demand for minimally invasive and faster recovery treatment options.
Europe minimally invasive spine surgery devices market accounted for USD 334.1 million in 2024.
- The market in Europe is influenced by several key factors, including the region's advanced healthcare infrastructure and its aging population. Both patients and surgeons are increasingly opting for minimally invasive procedures due to their benefits, such as reduced post-operative pain and shorter hospital stays.
- Reimbursement policies in many European countries further support the adoption of minimally invasive techniques. Additionally, ongoing clinical research and strong collaboration between medical device companies and healthcare providers are driving innovation and expanding procedural options across the region. Technological advancements, such as robotics and image-guided navigation, also play a crucial role in strengthening the market.
Germany is offering promising potentials in the minimally invasive spine surgery market.
- Germany, in particular, is experiencing significant market growth due to high surgical volumes and well-established orthopedic centers.
- Strong government investment in healthcare technologies and early adoption of advanced spinal systems, especially in urban areas and academic medical centers, further bolster the market in the country.
The Asia Pacific minimally invasive spine surgery devices market is anticipated to witness high growth over the analysis timeframe.
- The Asia Pacific market is expected to grow rapidly due to improving healthcare infrastructure, increasing awareness about advanced surgical options, and a growing elderly population prone to spinal disorders. Rising medical tourism in countries like India, Thailand, and South Korea also plays a key role, as these regions offer high-quality care at lower costs.
- Government initiatives aimed at modernizing hospitals and increasing access to specialized surgical care further contribute to market expansion. Additionally, the growing burden of lifestyle-related spinal conditions, such as herniated discs and degenerative spine diseases, continues to boost demand for effective and less invasive treatments.
China is expected to witness lucrative growth in the minimally invasive spine surgery devices market during the forecast period.
- In China, the market is propelled by a large patient pool, increased healthcare spending, and strong domestic manufacturing capabilities. Rapid urbanization and rising income levels are also enabling greater access to advanced spine care technologies.
- Further, China's rapidly aging population and increasing health awareness among the middle class are further driving factors for the adoption of minimally invasive spine surgery. According to the World Health Organization (WHO), the proportion of individuals aged 60 and above in China is projected to reach 28% by 2040, which is anticipated to significantly boost the demand for minimally invasive spine surgery procedures.
Brazil in the Latin America minimally invasive spine surgery devices market is expected to witness significant growth during the forecast period.
- The Brazilian public health system is under significant strain due to the increasing demand for advanced and expensive spine care solutions. According to an October 2024 report by the National Center for Biotechnology Information, approximately 25 million adults in Brazil are affected by back problems.
- Between 2010 and 2019, the Unified Health System (SUS) spent over USD 600 million addressing low back pain-related issues. To mitigate hospitalization costs, the government is actively promoting value-based care by encouraging the adoption of minimally invasive spinal surgical procedures, which is driving market growth.
Minimally Invasive Spine Surgery Devices Market Share
- The global market is highly consolidated, with leading companies such as Medtronic, Johnson & Johnson (DePuy Synthes), Stryker, Zimmer Biomet, and NuVasive collectively accounting for approximately 78% of the market share. These companies have established dominance through decades of innovation, extensive distribution networks, and strategic acquisitions, which have expanded their product portfolios and global presence. Their competitive edge is further strengthened by strong relationships with hospitals, ambulatory surgical centers, and spine specialists.
- These market leaders allocate significant resources to research and development, focusing on advancements such as enhanced imaging integration, robotic-assisted systems, and improved biocompatibility of implants. Additionally, their ability to scale manufacturing and navigate complex regulatory frameworks creates substantial entry barriers for smaller or newer competitors. Many of these companies also offer bundled solutions, including training, technical support, and service contracts, which enhance value for healthcare providers and foster customer loyalty.
- Meanwhile, mid-sized and emerging companies are gaining traction in specific regions or niche segments by introducing cost-effective or specialized products. Innovations such as biodegradable implants, navigation-assisted systems, and personalized surgical tools are helping these players carve out a presence in the market. However, their market penetration remains limited compared to established players due to challenges such as regulatory hurdles and lower brand recognition.
- In emerging markets, partnerships and local manufacturing collaborations are becoming critical strategies for expanding market access. While top-tier companies continue to dominate, the global MISS devices market is gradually opening up to innovation-driven entrants that differentiate themselves through pricing, innovation, or localized distribution. Nonetheless, factors such as brand reputation, clinician trust, and global logistics remain pivotal for maintaining leadership in this highly technical and evolving market segment.
Minimally Invasive Spine Surgery Devices Market Companies
Few of the prominent players operating in the minimally invasive spine surgery devices industry include:
- B. Braun
- DePuy Synthes (Johnson & Johnson)
- Evonik
- Globus Medical
- Heraeus
- Invibio
- Matexcel
- Medtronic
- Nexus Spine
- NuVasive
- Orthofix Medical
- Premia Spine
- SI-BONE
- Spinal Elements
- Stryker
- Wenzel Spine
- Xenco Medical
- Zimmer Biomet
- Medtronic
Medtronic leads the minimally invasive spine surgery devices market with a diverse portfolio that includes advanced navigation systems, robotics, and implants. The company’s strong emphasis on research and development, coupled with surgeon-focused innovations, enhances surgical precision and patient outcomes, solidifying its position as a market leader in image-guided and robotic-assisted spinal procedures.
- DePuy Synthes (Johnson & Johnson)
DePuy Synthes provides a comprehensive range of spinal implants and instruments designed for minimally invasive procedures. Its competitive edge lies in offering complete surgical solutions, including enabling technologies and surgeon education programs, which enhance procedural efficiency. The company’s extensive global network ensures reliable delivery and support across various healthcare settings.
- Stryker
Stryker is recognized for its innovative spinal navigation systems, power tools, and implant solutions tailored for minimally invasive spine surgeries. The company focuses on ergonomic designs and procedural adaptability, making its products suitable for both hospitals and ambulatory surgical centers. Its integrated platforms streamline surgical workflows and reduce operating times, contributing to its strong market presence.
- Zimmer Biomet
Zimmer Biomet specializes in precision-engineered, modular solutions for minimally invasive spine surgeries. Its devices are designed to simplify handling, minimize surgical exposure, and optimize instrument management. The company’s investments in digital technologies and data-driven platforms further enhance intraoperative planning and improve post-operative outcomes, reinforcing its position in the market.
Minimally Invasive Spine Surgery Devices Industry News:
- In April 2023, Orthofix Medical, a leading global spine and orthopedics company, announced the full commercial launch of two advanced access retractor systems designed to support surgeons in minimally invasive spine procedures. The Lattus Lateral Access System and the Fathom Pedicle-Based Retractor System enhance the company’s portfolio of access solutions within the minimally invasive spine surgery devices market.
- In October 2022, DePuy Synthes, the orthopedic division of Johnson & Johnson MedTech, secured FDA clearance for its TELIGEN System. This innovative platform is designed to support minimally invasive transforaminal lumbar interbody fusion (TLIF) procedures by integrating visualization and access tools, thereby improving precision and efficiency in lumbar decompression and fusion surgeries.
- In August 2022, Wenzel Spine, Inc., a medical technology company specializing in minimally invasive spine solutions, launched the S-LIF Procedure for stand-alone lumbar interbody fusion. This procedure leverages the company’s VariLift-LX device, aiming to streamline the fusion process while ensuring stability and reducing the need for supplemental fixation in specific cases.
- In July 2022, Nexus Spine, recognized for its advanced biomechanical spinal solutions, introduced the Stable-C cervical interbody fusion implants. These implants incorporate integrated anchoring blades to enhance implant stability and fixation, providing surgeons with a dependable option for treating cervical spine disorders while improving surgical outcomes and simplifying procedures.
The minimally invasive spine surgery devices market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million and from 2021 – 2034 for the following segments:
Market, By Product Type
- Implants and instrumentation
- Pedicle screws and rods
- Interbody cages
- Fixation systems
- Other implants and instrumentations
- Biomaterials
- Bone graft substitutes
- Synthetic bone grafts
- Other biomaterials
Market, By Application
- Fusion surgery
- Non-fusion surgery
Market, By Indication
- Lumbar disc herniation
- Spinal stenosis
- Degenerative spinal disease
- Cervical disc disorders
- Thoracic disc herniation
- Other indications
Market, By End Use
- Hospitals
- Ambulatory surgical centers
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- Saudi Arabia
- South Africa
- UAE
Frequently Asked Question(FAQ) :
What are the upcoming trends in the minimally invasive spine surgery devices industry?
Key trends include advancements in medical imaging and navigation technologies, increasing preference for minimally invasive procedures due to reduced recovery times and scarring, and a shift towards value-based care emphasizing shorter hospital stays.
Who are the key players in the minimally invasive spine surgery devices market?
Key players include B. Braun, DePuy Synthes (a Johnson & Johnson subsidiary), Evonik, Globus Medical, Heraeus, Invibio, and Matexcel.
Which region leads the minimally invasive spine surgery devices market?
North America led the market with a 35.3% share in 2024, driven by advanced healthcare infrastructure and high adoption of minimally invasive procedures.
What was the valuation of the biomaterials segment?
The biomaterials segment is projected to grow at a CAGR of 4.2%, driven by the demand for advanced bone graft substitutes that enhance spinal fusion and healing outcomes.
What is the market size of the minimally invasive spine surgery devices market in 2024?
The market size was USD 1.38 billion in 2024, with a CAGR of 5.1% expected through 2034, driven by the demand for smaller incisions, reduced hospital stays, faster recovery times, and advancements in medical imaging and navigation technologies.
What is the projected size of the minimally invasive spine surgery devices industry in 2025?
The market is expected to reach USD 1.42 billion in 2025.
How much revenue did the fusion surgery segment generate?
The fusion surgery segment accounted for 66.3% of the market share, driven by the rising prevalence of degenerative disc diseases and increasing demand for spinal stabilization procedures.
What is the projected value of the minimally invasive spine surgery devices market by 2034?
The market is expected to reach USD 2.22 billion by 2034, supported by increasing adoption of minimally invasive procedures and technological advancements enhancing precision and safety.
Minimally Invasive Spine Surgery Devices Market Scope
Related Reports


