Summary
Table of Content

Left Atrial Appendage Closure Devices Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Left Atrial Appendage Closure Devices Market Size
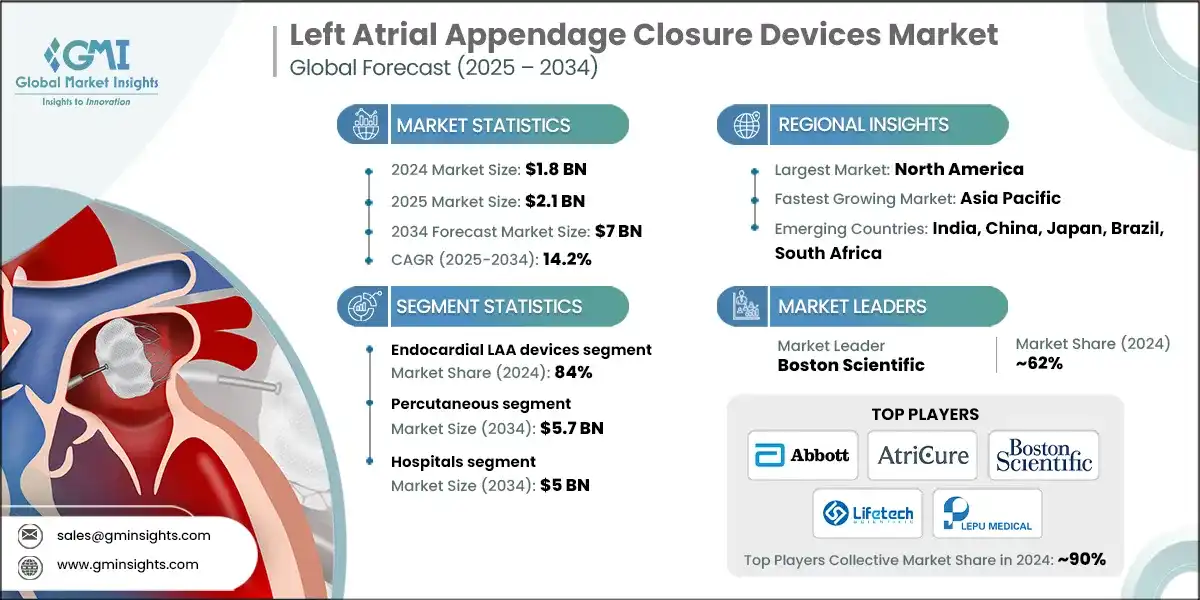
The global left atrial appendage closure devices market was valued at USD 1.8 billion in 2024. The market is expected to grow from USD 2.1 billion in 2025 to USD 7 billion in 2034, at a CAGR of 14.2% during the forecast period, according to the latest report published by Global Market Insights Inc. The high market growth is due to the increasing prevalence of atrial fibrillation, growing adoption of minimally invasive procedures, and expanding reimbursement coverage, among others.
To get key market trends
Left atrial appendage (LAA) closure devices help lower the risk of stroke in individuals with non-valvular atrial fibrillation by blocking off the left atrial appendage, which is a frequent site for blood clot formation. Key players include Boston Scientific, Abbott, AtriCure, Lifetech, and Medtronic, among others, each offering a range of endocardial and epicardial occlusion systems suited for both minimally invasive and surgical applications in clinical settings.
The market has increased from USD 1.3 billion in 2021 and reached USD 1.6 billion in 2023, with a historic growth rate of 11.4%. This growth was primarily driven by the rising prevalence of atrial fibrillation, increasing demand for minimally invasive cardiac procedures, expanding reimbursement coverage across developed markets, and growing clinical evidence supporting LAA closure as an effective alternative to long-term anticoagulation therapy.
Atrial fibrillation (AF) is the most prevalent arrhythmia, which is characterized by irregular and frequently accelerated rhythm of the heart, resulting in decreased blood circulation and the development of blood clots inside the heart. The prevalence of atrial fibrillation is increasing worldwide, especially in elderly populations. For instance, according to the Centers for Disease Control and Prevention (CDC), it is projected that 12.1 million U.S. individuals will have AFib by 2030. Increased prevalence results in a wider patient population that could be helped by LAA closure devices, thus fueling demand.
Additionally, governments across the globe are laying greater emphasis on financing cardiovascular health schemes, aware of the economic impact of atrial fibrillation and its resultant complications, such as stroke. Greater funding encourages research and development of new therapies, including LAA closure devices. Such financial support also induces large-scale public health initiatives to sensitize people toward AF and the advantages of early therapy using advanced cardiovascular devices.
Left atrial appendage closure devices are medical devices designed to prevent blood clots from forming in the left atrial appendage (LAA) of the heart, which can then cause strokes in patients with atrial fibrillation. These devices work by sealing off the LAA, reducing the risk of clot formation and subsequent stroke.
Left Atrial Appendage Closure Devices Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 1.8 Billion |
| Market Size in 2025 | USD 2.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 14.2% |
| Market Size in 2034 | USD 7 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of atrial fibrillation | Rising AF cases globally are driving demand for stroke-prevention alternatives like LAA closure. |
| Technological advancements | Innovations in device design and imaging are improving procedural safety and expanding clinical adoption. |
| Growing adoption of minimally invasive procedures | Preference for catheter-based interventions is accelerating market penetration across care settings. |
| Expanding reimbursement coverage | Broader insurance support is making LAA closure more accessible to eligible patients. |
| Pitfalls & Challenges | Impact |
| High procedural costs | Expensive devices and imaging systems limit adoption in cost-sensitive and emerging healthcare markets. |
| Risk of complications | Potential for device-related thrombus, incomplete closure, or procedural errors requires skilled operators and post-op care. |
| Opportunities: | Impact |
| Expansion into emerging markets | Rising atrial fibrillation burden and improving cardiac care infrastructure in regions like Asia-Pacific and Latin America offer strong growth potential. |
| Market Leaders (2024) | |
| Market Leaders |
~62% market share |
| Top Players |
Collective Market Share ~90% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Countries | India, China, Japan, Brazil, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Left Atrial Appendage Closure Devices Market Trends
The LAA closure devices market has experienced remarkable growth in recent years, largely propelled by the rapid pace of technological advancements.
- One of the most significant trends in the LAA closure devices market is the shift towards minimally invasive techniques. Recent developments have led to devices that can be implanted through smaller access points, minimizing trauma to patients. This not only enhances patient comfort and reduces the length of hospital stays but also facilitates faster recovery times. With improvements in device design and delivery systems, healthcare professionals are now able to perform LAA closures with greater ease and precision, ensuring better patient outcomes.
- Moreover, recent technological advancements have led to the development of more sophisticated and effective LAA closure devices. Modern devices are designed to conform better to the anatomical variations of the left atrial appendage, ensuring a more secure and complete closure. These improvements minimize the risk of residual leaks and embolization, thereby enhancing patient outcomes and reducing complications associated with the procedure.
- Additionally, the development of biocompatible materials minimizes adverse reactions and promotes seamless tissue integration. Devices constructed from advanced polymers and metals reduce inflammation and enhance stability, contributing to improved long-term patient outcomes. This focus on material science is pivotal in ensuring that the benefits of LAA closure are realized not just immediately, but throughout the patient’s post-procedure life.
Left Atrial Appendage Closure Devices Market Analysis
Learn more about the key segments shaping this market
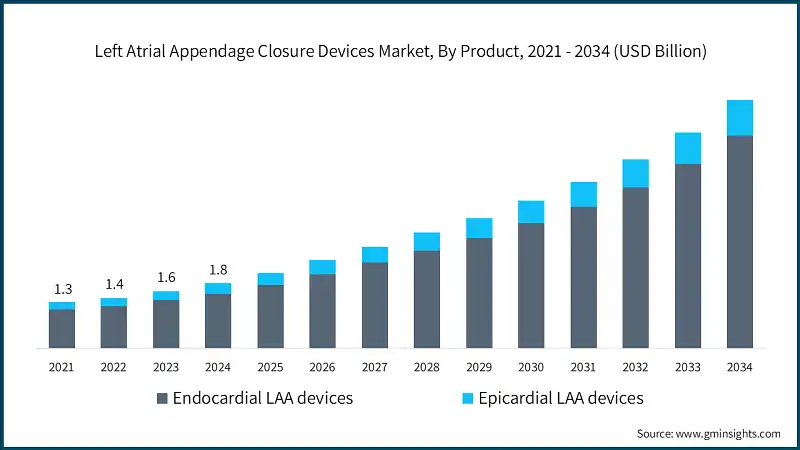
The global market was valued at USD 1.3 billion in 2021. The market size reached USD 1.6 billion in 2023, from USD 1.4 billion in 2022.
Based on the product, the market is segmented into endocardial LAA devices and epicardial LAA devices. The endocardial LAA devices segment accounted for 84% of the market in 2024 due to their minimally invasive nature, high procedural success rates, extensive clinical validation, and widespread physician preference for percutaneous approaches in stroke prevention for atrial fibrillation patients. The segment is expected to exceed USD 6 billion by 2034, growing at a CAGR of 14.4% during the forecast period.
On the other hand, the epicardial LAA devices segment held a market share of 16% in 2024. The growth of this segment can be attributed to its suitability for patients undergoing open-heart surgery, its effectiveness in cases where endocardial access is limited or contraindicated, and the increasing adoption of hybrid procedures that combine surgical ablation with epicardial LAA closure.
- Endocardial LAA devices have shown high safety and efficacy in clinical trials and real-world use. The device minimize the stroke risk in patients with atrial fibrillation by sealing off the left atrial appendage, where blood clots tend to form. The strong clinical evidence on the use of endocardial devices has contributed to the growing confidence among providers and widespread acceptance in clinical practice.
- Additionally, most patients are inclined towards endocardial LAA closure devices because the procedure is minimally invasive and has attached advantages, including less pain and quick recovery. The positive outcomes and experiences of the patient when using endocardial devices have made them more popular. This awareness among patients and providers will lead to increased market share for this segment over the analysis timeframe.
Based on procedure, the left atrial appendage closure devices market is segmented into percutaneous and surgical. The percutaneous segment is projected to reach USD 5.7 billion by 2034.
- Percutaneous LAA closure procedures are widely accessible and can be performed in a variety of healthcare settings, including hospitals and specialized cardiac centers. The availability of trained interventional cardiologists and electrophysiologists, combined with advanced catheterization labs, ensures that a broad range of patients can benefit from these procedures. The wide accessibility of percutaneous techniques contributes to their market dominance.
- Additionally, percutaneous LAA closure procedures are cost-effective compared to traditional surgical approaches. The reduced need for extended hospital stays, lower risk of complications, and faster recovery times contribute to overall cost savings for healthcare systems. The economic benefits of percutaneous procedures make them an attractive option for both patients and healthcare providers, further supporting their market dominance.
- The surgical segment was valued at approximately USD 376.5 million in 2024, driven by its use in patients undergoing open-heart procedures, increasing adoption of hybrid ablation and closure techniques, and growing clinical evidence supporting surgical LAA closure as an effective stroke prevention strategy for atrial fibrillation patients who are not candidates for percutaneous interventions.
Learn more about the key segments shaping this market
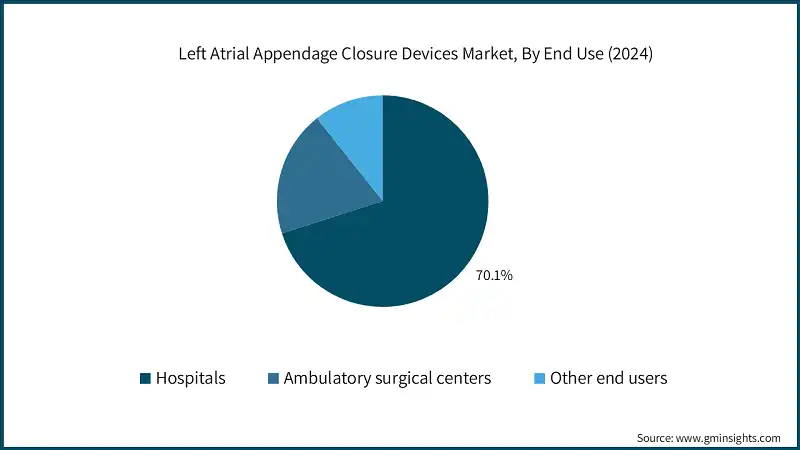
Based on end use, the left atrial appendage closure devices market is segmented into hospitals, ambulatory surgical centers, and other end users. The hospitals segment dominated the market with a revenue share of 70.1% in 2024 and is expected to reach USD 5 billion within the forecast period.
- Hospitals are equipped with comprehensive facilities and advanced medical technologies necessary for performing complex LAA closure procedures. They have state-of-the-art catheterization labs, imaging equipment, and surgical suites that support both minimally invasive and surgical approaches to LAA closure. This infrastructure enables hospitals to handle a high volume of procedures, ensuring that patients receive the best possible care.
- The ambulatory surgical centers segment was valued at approximately USD 354.6 million in 2024, driven by the growing shift toward outpatient cardiac procedures, reduced procedural costs compared to hospital settings, faster patient recovery times, and increasing adoption of minimally invasive LAA closure techniques in specialized cardiac centers.
- On the other hand, the other end users segment held a market share of 10.7% in 2024. The growth of this segment can be attributed to the increasing adoption of LAA closure procedures in specialized cardiac clinics, research institutions, and long-term care facilities that fall outside traditional hospital and ambulatory surgical settings.
Looking for region specific data?
North America dominated the global left atrial appendage closure devices market with the highest market share of 46.2% in 2024.
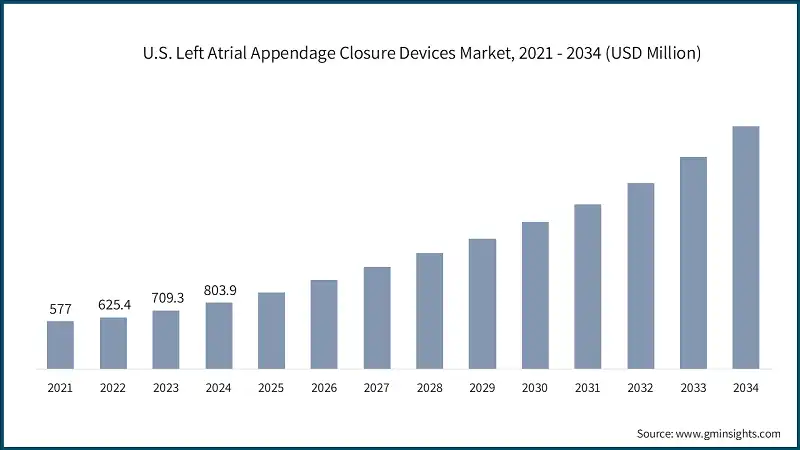
- The U.S. left atrial appendage closure devices industry was valued at USD 577 million and USD 625.4 million in 2021 and 2022, respectively. The market size reached USD 803.9 million in 2024, growing from USD 709.3 million in 2023, and is anticipated to grow at a CAGR of 13.7% between 2025 and 2034.
- North America holds a dominant position in the global left atrial appendage closure devices industry, driven by a high prevalence of atrial fibrillation, advanced healthcare infrastructure, and strong clinical adoption of minimally invasive cardiac procedures. The U.S. is the primary contributor, with widespread use of FDA-approved devices like Boston Scientific’s WATCHMAN and Abbott’s Amplatzer Amulet. The region also benefits from robust reimbursement policies under Medicare and private insurers, which significantly improve patient access to these therapies.
- Additionally, North America is a hub for clinical research and innovation in structural heart interventions. Leading academic centers and hospitals frequently participate in trials that validate the safety and efficacy of LAA closure devices, further accelerating market growth. Physician training programs, digital health integration, and AI-assisted procedural planning tools are also more prevalent in this region, reinforcing its technological edge and procedural volume.
Europe left atrial appendage closure devices market accounted for USD 489.1 million in 2024.
- The region’s growth is fueled by a rapidly aging population, high AF diagnosis rates, and favorable regulatory pathways such as CE Mark approvals. Countries like Germany, France, the UK, and Italy are key markets, with increasing adoption in both public and private healthcare systems. European cardiology societies have also incorporated LAA closure into clinical guidelines, supporting broader procedural adoption.
- Moreover, Europe is seeing a rise in hybrid cardiac procedures, where LAA closure is performed alongside ablation or valve repair. The presence of well-established cardiac centers and a strong focus on minimally invasive techniques are helping drive demand.
The Asia Pacific left atrial appendage closure devices market is anticipated to grow at the highest CAGR of 15.3% during the analysis timeframe.
- This growth is driven by increasing AF prevalence, rising awareness of stroke prevention, and improving access to advanced cardiac care. Countries like China, Japan, India, South Korea, and Australia are emerging as key markets. Local manufacturers are also contributing to regional growth by offering cost-effective alternatives to Western devices.
- Government investments in healthcare infrastructure, especially in China and India, are enabling more hospitals to adopt structural heart interventions. Additionally, the region is seeing a surge in medical tourism and private cardiac centers, which are more likely to offer LAA closure procedures. Despite strong growth potential, challenges such as regulatory delays and limited reimbursement in some countries may slow adoption.
The Latin America left atrial appendage closure devices market is expected to experience robust growth over the analysis timeframe.
- Brazil and Mexico are the leading countries in the region, supported by growing awareness of AF-related stroke risks and gradual improvements in cardiac care infrastructure. The adoption of minimally invasive procedures is increasing, particularly in private hospitals and specialty clinics.
- However, the region faces several barriers, including limited reimbursement coverage, high device costs, and uneven access to trained interventional cardiologists. Despite these challenges, international manufacturers are expanding their presence through partnerships and training programs, which could accelerate market growth in the coming years.
The Middle East and Africa (MEA) market is expected to experience notable growth over the analysis timeframe.
- Countries like the UAE, Saudi Arabia, and South Africa are leading adoption, driven by rising AF diagnosis rates and investments in tertiary cardiac care centers. The region is increasingly focused on modernizing healthcare systems and attracting global medtech companies.
- While procedural volumes remain low compared to other regions, MEA offers long-term growth potential due to its expanding middle class, rising healthcare expenditure, and strategic focus on non-communicable diseases. Challenges such as limited specialist availability, regulatory hurdles, and affordability issues still need to be addressed to unlock full market potential.
Left Atrial Appendage Closure Devices Market Share
Leading industry players such as Abbott, AtriCure, Boston Scientific, LEPU MEDICAL, and Lifetech hold around 90% of the market share in the consolidated market. These companies maintain their leading position by combining strong product lines, business collaborations with healthcare providers, regulatory clearances, and consistent product innovation.
Boston Scientific continues to lead the global market with its flagship WATCHMAN product line, including the advanced WATCHMAN FLX and FLX Pro. These heart closure devices are widely adopted due to their proven safety, ease of deployment, and compatibility with a broad range of LAA anatomies.
Abbott has established a strong competitive position with its Amplatzer Amulet LAA Occluder, known for its dual-seal technology that enables immediate closure and minimizes the need for post-procedural anticoagulation.
Meanwhile, other key players such as AtriCure, Lifetech Scientific, and Lepu Medical are reinforcing their market presence through specialized innovations, regional expansion, and advanced procedural solutions.
Left Atrial Appendage Closure Devices Market Companies
A few of the prominent players operating in the left atrial appendage closure devices industry include:
- Abbott
- AtriCure
- Boston Scientific
- LEPU MEDICAL
- Lifetech
- Medtronic
- MicroPort
- Abbott
Abbott distinguishes itself through its deep expertise in structural heart therapies and its commitment to delivering clinically validated solutions for stroke prevention in atrial fibrillation patients. The company’s global cardiology network and strong presence in both developed and emerging markets enable widespread adoption of its LAA closure technologies. Abbott’s focus on procedural efficiency, physician education, and integration with broader cardiac care pathways reinforces its reputation as a trusted partner in interventional cardiology.
Boston Scientific leads the market with a share of 62% in 2024. Boston Scientific maintains a leading position in the LAA closure devices market by combining innovation, clinical evidence, and physician-centric design. Its solutions are widely recognized for their anatomical versatility, procedural simplicity, and strong safety profile. The company’s investment in digital planning tools, post-procedural monitoring, and global training programs supports consistent outcomes and high procedural volumes across diverse healthcare settings, making it a preferred choice for stroke prevention in AF patients.
AtriCure brings a unique surgical perspective to the LAA closure market, offering specialized solutions tailored for patients undergoing open-heart procedures or hybrid cardiac interventions. Its strength lies in combining LAA exclusion with surgical ablation strategies, addressing complex AF cases with a comprehensive approach. AtriCure’s focus on clinical education, long-term efficacy, and integration into electrophysiology workflows positions it as a key innovator in the surgical segment of the LAA closure landscape.
Left Atrial Appendage Closure Devices Industry News:
- In November 2023, Medtronic announced the U.S. launch of its Penditure Left Atrial Appendage Exclusion System, following FDA clearance. This innovative surgical solution is designed to support stroke risk reduction in patients with atrial fibrillation undergoing cardiac surgery. This product launch helped the company strengthen its position in the LAA closure devices market.
- In September 2023, Boston Scientific Corporation announced it had received U.S. Food and Drug Administration approval for the latest-generation WATCHMAN FLX Pro Left Atrial Appendage Closure (LAAC) Device. This approval may help the company to enhance its product portfolio and offerings to customers.
The left atrial appendage closure devices market research report includes an in-depth coverage of the industry with estimates and forecasts in terms of revenue in USD Million and units in Volume from 2021 - 2034 for the following segments:
Market, By Product
- Endocardial LAA devices
- Epicardial LAA devices
Market, By Procedure
- Percutaneous
- Surgical
Market, By End Use
- Hospitals
- Ambulatory surgical centers
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Who are the key players in the left atrial appendage closure devices market?
Key players include Boston Scientific, Abbott, AtriCure, Lifetech, LEPU Medical, Medtronic, and MicroPort, with Boston Scientific leading at ~62% market share in 2024.
Which region leads the left atrial appendage closure devices market?
North America led the market with 46.2% share and USD 803.9 million in 2024, supported by strong clinical adoption, advanced healthcare infrastructure, and favorable reimbursement policies.
How much revenue did hospitals generate in 2024?
Hospitals generated about 70.1% of total revenue in 2024, valued at nearly USD 1.3 billion, owing to advanced infrastructure, catheterization labs, and higher procedural volumes.
Which product segment dominated the market in 2024?
Endocardial LAA devices dominated with 84% market share in 2024 due to their minimally invasive nature, high procedural success rates, and strong clinical validation. The segment is projected to surpass USD 6 billion by 2034.
What was the valuation of the surgical procedure segment in 2024?
The surgical LAA closure segment was valued at approximately USD 376.5 million in 2024, supported by hybrid cardiac procedures and its use in open-heart surgery patients.
What is the market size of the left atrial appendage closure devices market in 2024?
The market size was USD 1.8 billion in 2024, driven by the rising prevalence of atrial fibrillation, adoption of minimally invasive procedures, and expanding reimbursement coverage.
What is the estimated market size of left atrial appendage closure devices in 2025?
The market is projected to reach USD 2.1 billion in 2025, reflecting strong early growth momentum.
What is the projected value of the left atrial appendage closure devices market by 2034?
The market is expected to reach USD 7 billion by 2034, growing at a CAGR of 14.2% during 2025–2034, supported by technological advancements and broader clinical adoption.
Left Atrial Appendage Closure Devices Market Scope
Related Reports


