Summary
Table of Content

Drug Repurposing Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Drug Repurposing Market Size
The global drug repurposing market was valued at USD 32.6 billion in 2024. The market is expected to grow from USD 33.8 billion in 2025 to USD 51.1 billion in 2034, at a CAGR of 4.7% during the forecast period according to the latest report published by Global Market Insights Inc. This growth is attributed to the increasing incidence of conditions such as cancer, Alzheimer’s, and rare genetic disorders fueling demand for faster therapeutic solutions.

To get key market trends
Repurposed drugs offer quicker access to therapies, especially for rare diseases where traditional research and development is limited due to low commercial incentives. For instance, cariprazine was repurposed for major depressive disorder, and empagliflozin was approved for chronic kidney disease, demonstrating the growing trend of using existing drugs to address unmet medical needs.
Additionally, drug repurposing significantly reduces the cost and duration of drug development. Traditional drug discovery can take over a decade and cost upward USD 2 billion. In contrast, repurposing uses drugs with known safety profiles, bypassing early-stage trials and accelerating regulatory approval. This approach is especially attractive to pharmaceutical companies seeking faster returns on investment, that further contributes to market growth.
Drug repurposing is the process of finding new therapeutic uses for existing drugs, which were originally developed for a different medical condition. This strategy can reduce the time and cost of drug development because the safety and pharmacological profiles of the drugs are already known. It involves identifying and testing drugs for indications other than their original one, with examples including repurposing approved, discontinued, or abandoned drugs. Major players in the industry are Recursion Pharmaceuticals, Pfizer, Novartis, Eli Lilly and Company and Melior Discovery. These players dominated the market by adopting various strategies such as product expansion and establishing global distribution networks.
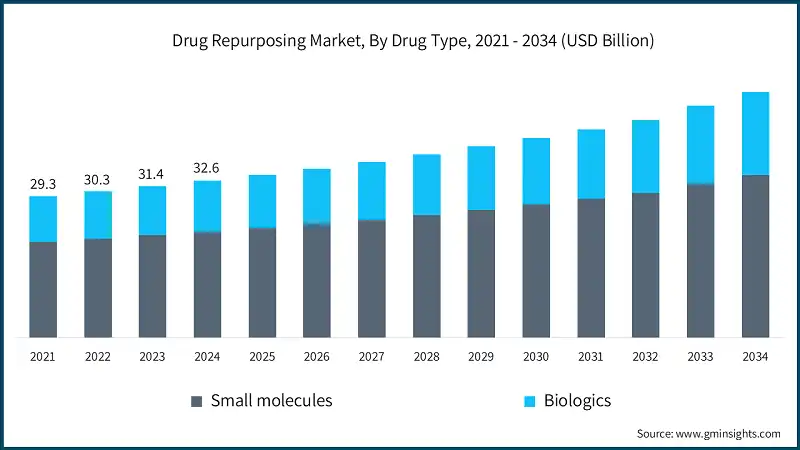
Drug repurposing market has witnessed steady growth, growing from USD 29.3 billion in 2021 to USD 31.4 billion in 2023. AI and machine learning are transforming drug repurposing by enabling rapid identification of new therapeutic uses for existing compounds. Platforms like Every Cure’s MATRIX, powered by Google Cloud’s Gemini 2.0, use large language models and big data analytics to accelerate discovery and validation.
These technologies improve prediction accuracy, reduce trial-and-error, and enhance scalability. Computational modeling and GPU-based cloud computing are also being used to simulate drug-protein interactions, making repurposing more efficient and data-driven. This tech-driven approach is fostering innovation and expanding treatment options for diseases with limited therapies, fueling the growth of the market.
Moreover, regulatory agencies are actively supporting drug repurposing through streamlined approval pathways. The FDA’s 505(b)(2) and EMA’s adaptive licensing frameworks allow companies to use existing safety and efficacy data, reducing the need for full clinical trials. This not only lowers development costs but also speeds up time-to-market. Such incentives are crucial for addressing urgent medical needs and encouraging innovation.
Drug Repurposing Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 32.6 Billion |
| Market Size in 2025 | USD 33.8 Billion |
| Forecast Period 2025 - 2034 CAGR | 4.7% |
| Market Size in 2034 | USD 51.1 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising prevalence of chronic and rare diseases | The increasing burden of diseases like cancer, CKD, and MDD is fueling demand for repurposed drugs. |
| Cost and time efficiency of drug repurposing | Drug repurposing significantly reduces research and development costs and timelines. This efficiency makes it attractive for pharma companies aiming to minimize risk and maximize ROI. |
| Integration of AI-driven platforms | These tools analyze vast datasets to identify new therapeutic uses for existing drugs. |
| Unmet medical needs and drug shortages | Repurposing offers a strategic solution for rare and neglected diseases, which often lack dedicated research and development due to low commercial incentives. |
| Pitfalls & Challenges | Impact |
| Lack of incentives for off-patent drugs | Repurposing generic or off-patent drugs offers limited financial returns due to lack of exclusivity. |
| Liability and safety concerns | Repurposing drugs for new indications may expose companies to legal risks if adverse effects arise outside the original scope. |
| Opportunities: | Impact |
| Growing demand for cost-effective therapies | With drug development costs exceeding USD 2.6 billion, repurposing offers a faster, cheaper alternative. |
| Expansion into rare and neglected diseases | Repurposing is ideal for diseases with limited research and development funding. It provides treatment options for underserved populations, especially in Asia-Pacific, the fastest-growing region. |
| Market Leaders (2024) | |
| Market Leaders |
~12.4% market share. |
| Top Players |
Collective market share in 2024 is ~35% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Emerging Country | China, India, Brazil, Mexico, South Africa |
| Future Outlook |
|
What are the growth opportunities in this market?
Drug Repurposing Market Trends
- The market is expanding due to rising chronic disease prevalence, aging populations, and the urgent need for cost-effective therapies. With over 14,000 diseases lacking approved treatments, repurposing offers a faster route to address unmet medical needs. North America leads due to strong regulatory frameworks, while Asia-Pacific is the fastest-growing region, benefiting from lower clinical trial costs and expanding infrastructure.
- In addition, smaller biotech firms and academic institutions are increasingly driving innovation through AI-powered platforms and real-world evidence solutions. Rare and orphan diseases are gaining traction due to favorable incentives and venture funding. Drug-centric and disease-centric approaches dominate, with oncology and CNS disorders leading therapeutic areas, that further contributes to market growth.
- Moreover, repurposed drugs are increasingly tailored for specific indications, with oncology and rare diseases leading. Small molecules dominate, but biological research is gaining ground due to falling production costs. Combination therapies and personalized medicine approaches are also emerging.
- The increasing venture funding and strategic collaborations with growing investment in drug repurposing, with biotech startups and academic institutions forming alliances with pharma giants. Venture capital is flowing into AI-driven platforms and rare disease research. For example, Every Cure’s partnership with Google Cloud exemplifies how tech and pharma are converging to accelerate repurposing. These collaborations are fostering innovation and expanding the scope of repurposing beyond traditional therapeutic areas.
- Regulators are increasingly accepting RWE in drug approval processes, especially for repurposed drugs. This includes data from electronic health records, patient registries, and observational studies. RWE helps validate efficacy and safety in real-world settings, reducing the need for extensive clinical trials. The FDA and EMA are updating frameworks to incorporate RWE, making repurposing more viable for rare and underserved conditions.
- Further, innovative business models are emerging to address IP challenges in repurposing. Nonprofits and public-private partnerships are exploring “labeling-only” pathways to repurpose generics without full commercialization. Meanwhile, pharma companies are using lifecycle extension strategies to maintain revenue from expiring patents.
- Lastly, beyond traditional drug-centric approaches, companies are adopting disease-centric and target-centric models. These methods focus on understanding disease mechanisms and biological targets to identify suitable existing drugs. This shift is enhancing success rates and expanding repurposing into rare and neglected diseases, which often lack commercial incentives for new drug development.
Drug Repurposing Market Analysis
Learn more about the key segments shaping this market
Based on drug type, the global drug repurposing market is divided into small molecules and biologics. The small molecules segment accounted for 67.5% of the market in 2024. The segment is expected to exceed USD 33.8 billion by 2034, growing at a CAGR of 4.5% during the forecast period.
- Small molecules have well-documented pharmacokinetics, safety profiles, and manufacturing processes, making them ideal candidates for repurposing. Their prior approval allows developers to leverage existing clinical data, reducing the need for extensive trials. Regulatory frameworks like the FDA’s 505(b)(2) pathway enable faster approvals by referencing prior data, significantly cutting time and cost.
- Small molecules are easier and cheaper to synthesize compared to biologics. Their chemical stability and simpler production processes allow for large-scale manufacturing using existing infrastructure. This cost-efficiency is crucial for repurposing, where rapid deployment is often needed.
- Additionally, their compatibility with combination therapies and potential for oral administration enhances patient compliance and market adoption. This broad applicability supports continuous innovation and expansion of repurposing pipelines.
- The biologics segment of the drug repurposing market is projected to grow significantly with a CAGR of 5.1%. Biologics, including monoclonal antibodies, recombinant proteins, and gene therapy, offer high specificity and efficacy in targeting disease mechanisms. This makes them ideal for repurposing g in complex conditions like cancer, autoimmune disorders, and neurological diseases.
- Moreover, their ability to interact with specific cellular pathways allows for precision treatment, reducing off-target effects. For instance, biologics such as Leqembi (lecanemab) have been repurposed for Alzheimer’s disease, showing promising results in slowing cognitive decline.
- Thus, as biologics continue to demonstrate superior therapeutic outcomes, their repurposing potential grows, especially in areas with unmet medical needs.
Based on the therapeutic area, the drug repurposing market is segmented into oncology, neurology and CNS disorders, infectious diseases, cardiovascular diseases, metabolic disorders, and other therapeutic areas. The oncology segment dominated the market in 2024 with a revenue of USD 12.3 billion.
- The increasing prevalence of cancer worldwide is a major driver for oncology-focused drug repurposing. According to the American Cancer Society, over 2 million new cancer cases were reported in the U.S. alone in 2024. This surge in diagnoses across all age groups has intensified the demand for effective and affordable treatments.
- Drug repurposing offers a faster route to address this need by utilizing existing drugs with known safety profiles. It enables quicker access to therapies, especially for aggressive cancers such as lung, breast, and colorectal, where time-sensitive intervention is critical. This urgency is pushing pharmaceutical companies to explore repurposing as a strategic solution, thereby driving the growth of the market.
- In addition, government initiatives and public-private partnerships are fueling oncology drug repurposing. For instance, Every Cure received a USD 48.3 million federal contract from ARPA-H in 2024 to support repurposing efforts for cancer and other diseases.
- These incentives, combined with rising healthcare expenditures and global awareness campaigns, are encouraging pharmaceutical companies to invest in repurposing strategies that further contribute to market growth.
- The neurology and CNS disorders segment of the drug repurposing market is projected to grow significantly during the analysis period owing to increasing global burden of neurological and psychiatric conditions such as Alzheimer’s, Parkinson’s, epilepsy, depression, and schizophrenia.
- Additionally, growing focus on mental health and societal awareness leads to increasing diagnosis and demand for effective treatments for conditions such as depression, anxiety, bipolar disorder, and schizophrenia. This societal shift is influencing healthcare policies, insurance coverage, and pharmaceutical priorities thereby boosting market growth.
Based on route of administration, the drug repurposing market is classified into oral, intravenous, and other routes of administration. The oral segment dominated the market in 2024 with USD 18.4 billion.
- Oral drugs are widely applicable across multiple therapeutic areas, including oncology, CNS disorders, infectious diseases, and metabolic conditions. Their ability to deliver systemic effects makes them suitable for treating both acute and chronic illnesses.
- In drug repurposing, oral formulations are often prioritized due to their versatility and established pharmacokinetic profiles. For example, many antiviral and anticancer agents originally developed for one indication have been successfully repurposed for others using oral delivery. This broad applicability enhances the commercial viability of repurposed oral drugs and supports their continued dominance in the market.
- The intravenous segment grew with a CAGR of 5.1% during the analysis period. Biologics, including monoclonal antibodies and recombinant proteins, are often administered intravenously due to their molecular complexity and sensitivity to digestion.
- The widespread availability of hospital infrastructure for IV drug administration supports the growth of this segment. Clinical trials for repurposed drugs often begin in controlled environments, and IV delivery is preferred for its dosing accuracy and monitoring capabilities.
Learn more about the key segments shaping this market
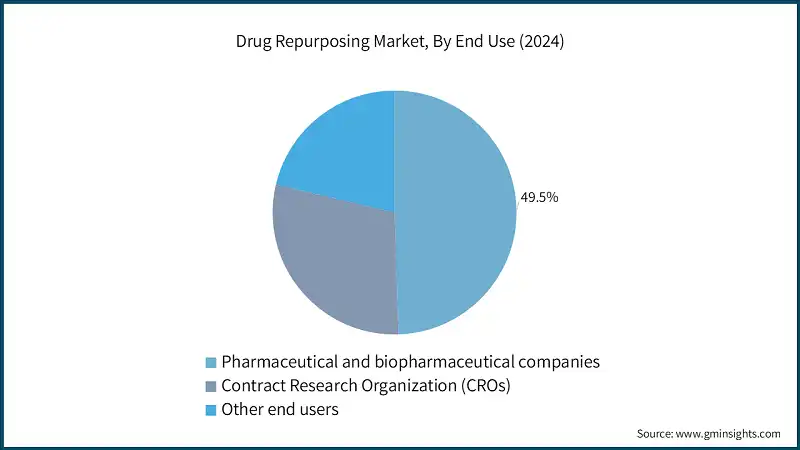
Based on end use, drug repurposing market is classified into pharmaceutical and biotechnology companies, contract research organization (CROs) and other end users. The pharmaceutical and biotechnology companies segment dominated the market in 2024 and is expected to reach USD 25.1 billion by 2034.
- Pharmaceutical and biotech companies are increasingly turning to drug repurposing to reduce the high costs and risks associated with traditional drug development. Repurposing allows them to leverage existing compounds with known safety profiles, significantly cutting down on preclinical and early-stage trial expenses.
- Pharmaceutical and biotech companies are increasingly adopting AI, machine learning, and big data analytics to identify new indications for existing drugs. These technologies enable faster target identification, lead optimization, and clinical trial design, boosting market share for this segment.
- Drug repurposing enables pharmaceutical and biotech companies to stay competitive by rapidly responding to emerging health threats and unmet medical needs. The ability to bring therapies to market faster than traditional research and development gives companies a strategic edge. For example, during the COVID-19 pandemic, repurposed drugs like remdesivir and dexamethasone were deployed quickly, demonstrating the value of this approach.
Looking for region specific data?
North America Drug Repurposing Market
The North America market dominated the market with a market share of 48.2% in 2024.
- North America, particularly the U.S., benefits from a well-established regulatory infrastructure that supports drug repurposing. The FDA’s 505(b)(2) pathway allows companies to use existing safety and efficacy data to gain approval for new indications, significantly reducing development time and cost. This regulatory flexibility encourages pharmaceutical and biotech firms to invest in repurposing strategies.
- North America has significant public and private investment in drug repurposing research. The National Institutes of Health (NIH) allocated USD 1.15 billion through the RECOVER initiative in 2021.
- Additionally, the NIH's National Center for Advancing Translational Sciences (NCATS) dedicated USD 8.2 million in 2022 specifically for drug repurposing research. The funding is projected to reach USD 2.5 billion by 2025, particularly focusing on responses to public health emergencies.
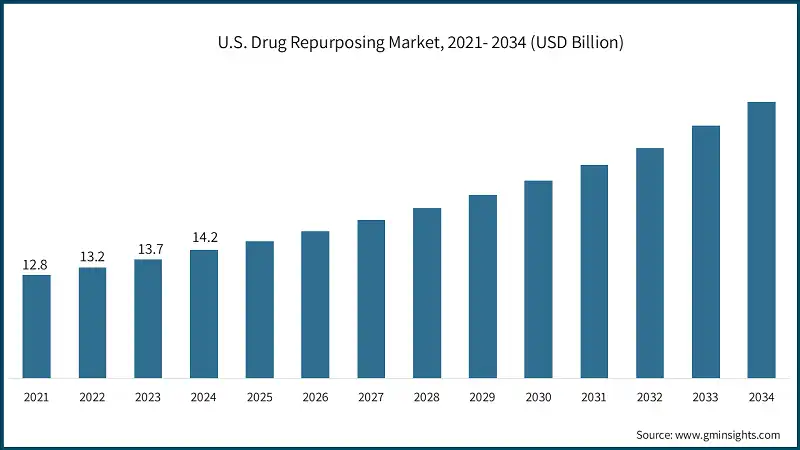
The U.S. drug repurposing market was valued at USD 12.8 billion and USD 13.2 billion in 2021 and 2022, respectively. The market size reached USD 14.2 billion in 2024, growing from USD 13.7 billion in 2023.
- The U.S. maintains one of the highest healthcare expenditures globally, accounting for 18.3% of GDP in 2021, according to the Centers for Medicare and Medicaid Services (CMS). National health spending is projected to reach USD 6.2 trillion by 2025. Drug repurposing reduces research and development costs while accelerating time-to-market, with the FDA reporting a 60% reduction in development timelines for repurposed drugs between 2021-2023.
- This approach is especially beneficial for managing chronic conditions such as diabetes, cancer, and cardiovascular diseases, which require extended treatment periods and collectively account for 75% of the nation's annual healthcare costs, as reported by the CDC in 2023.
- The U.S. is home to a robust research ecosystem comprising leading pharmaceutical companies, biotech startups, academic institutions, and government agencies. Collaborations between these entities are fueling innovation in drug repurposing.
Europe Drug Repurposing Market
Europe market accounted for USD 8.2 billion in 2024 and is anticipated to show lucrative growth over the forecast period.
- The European Medicines Agency (EMA) has introduced adaptive licensing and other flexible regulatory pathways that support drug repurposing. These frameworks allow companies to use existing safety and efficacy data to gain approval for new indications, reducing the need for full clinical trials. This is particularly beneficial for rare diseases and urgent therapeutic needs.
- The EMA also encourages real-world evidence and post-marketing surveillance, which helps validate repurposed drugs in broader populations. These regulatory incentives make Europe a favorable region for pharmaceutical and biotech firms to pursue repurposing strategies.
- Europe has a robust network of academic institutions, public health agencies, and research organizations actively involved in drug repurposing. These entities collaborate with pharmaceutical companies and biotech firms to identify new uses for existing drugs. EU-funded programs and Horizon Europe initiatives support cross-border research and innovation in drug repurposing.
Germany dominates the European drug repurposing market, showcasing strong growth potential.
- Germany is home to leading pharmaceutical and biotech companies such as Bayer, Merck KGaA, and Boehringer Ingelheim, which possess extensive drug portfolios and research and development capabilities. These firms are actively exploring repurposing opportunities to extend product lifecycles and enter new therapeutic areas. The country’s industrial strength supports innovation through advanced manufacturing, regulatory expertise, and global distribution networks, driving market growth.
- Germany's research infrastructure includes institutions such as the Max Planck Society, Fraunhofer Institutes, and universities. According to the Federal Ministry of Education and Research (BMBF), Germany invested EUR 104.7 billion in research and development in 2021, with 15% allocated to pharmaceutical research.
- The German government's 2025 Research and Innovation strategy aims to increase research and development spending to 3.5% of GDP, with EUR 25 billion specifically dedicated to drug development and repurposing initiatives. The partnerships between academic institutions and pharmaceutical companies support early-stage discovery and validation, which reduces the risks associated with drug repurposing projects. The German Research Foundation (DFG) reported funding 387 drug repurposing projects between 2021-2023, with a success rate of 42% in identifying viable new applications.
Asia Pacific Drug Repurposing Market
The Asia Pacific market is anticipated to grow at the highest CAGR of 5% during the analysis timeframe.
- Governments across Asia Pacific are implementing supportive policies and reforms to encourage pharmaceutical innovation, including drug repurposing. For example, China has streamlined its drug approval process, while Japan offers subsidies to boost local production. The region’s evolving regulatory landscape is creating a more favorable environment for faster approvals and commercialization of repurposed drugs.
- Asia Pacific has established itself as a global pharmaceutical manufacturing hub, with India and China dominating generic drug production. According to the Indian Ministry of Commerce and Industry, India's pharmaceutical exports reached USD 24.6 billion in FY 2021-22, with generic drugs accounting for 70% of the total exports.
- In addition, China's National Medical Products Administration reported that the country's pharmaceutical manufacturing output value reached CNY 3.4 trillion (USD 534 billion) in 2021. This manufacturing capacity allows quick deployment of repurposed therapies, particularly during health emergencies or drug shortages, contributing to market growth.
China drug repurposing market is estimated to grow with a significant CAGR, in the Asia Pacific market.
- The Chinese government has actively promoted drug repurposing as part of its broader healthcare reform strategy. Policies encouraging innovation, faster access to essential medicines, and improved patient outcomes have created a favorable environment for repurposing efforts. Funding programs and regulatory incentives are being introduced to support pharmaceutical research and development, especially for rare and chronic diseases.
- Collaborations between pharmaceutical companies and academic institutions in China are accelerating drug repurposing efforts. Universities and research centers contribute to early-stage discovery, while industry partners handle clinical development and commercialization. These partnerships are supported by government grants and innovation hubs, fostering a collaborative ecosystem for repurposing research.
Latin American Drug Repurposing Market
Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period.
- Brazil’s pharmaceutical sector is increasingly adopting drug repurposing as a cost-effective alternative to traditional drug discovery. Developing new drugs from scratch is expensive and time-consuming, often taking over a decade and billions in investment.
- Repurposing allows Brazilian companies to bypass early-stage trials by leveraging existing safety and efficacy data, significantly reducing research and development costs. This is particularly beneficial in Brazil’s public healthcare system (SUS), which seeks affordable treatment options for widespread diseases. As healthcare budgets tighten, repurposing offers a strategic solution to deliver effective therapies faster and at lower costs.
- Brazil faces a growing prevalence of chronic conditions such as cancer, cardiovascular diseases, and diabetes, alongside persistent infectious diseases like dengue and Zika. These health challenges often lack effective treatments, making drug repurposing a valuable strategy.
Middle East and Africa Drug Repurposing Market
Saudi Arabia market to experience substantial growth in the Middle East and Africa market in 2024.
- Saudi Arabia is rapidly building its pharmaceutical manufacturing capabilities, supported by local firms like SPIMACO and Tabuk Pharmaceuticals. These companies are investing in proprietary formulations and repurposing pipelines to move up the value chain.
- The development of research-driven pharmaceutical zones and innovation hubs is enhancing the country’s capacity for drug discovery and repurposing. This infrastructure growth supports faster production, clinical validation, and commercialization of repurposed drugs, reducing reliance on imports and improving supply chain resilience.
- With rising healthcare costs and a growing population, Saudi Arabia is prioritizing cost-effective treatment options. Drug repurposing offers a faster and more economical route to bring therapies to market, especially for diseases with limited treatment options. Hospitals and healthcare providers are increasingly adopting repurposed drugs to manage patient loads efficiently.
Drug Repurposing Market Share
The market is characterized by a dynamic and moderately fragmented competitive environment. Leading pharmaceutical and biotech companies are actively shaping the market through strategic investments in research and development, expansion into emerging regions, and the creation of patient-centric therapies targeting complex conditions such as cancer, autoimmune disorders, and infectious diseases. The key players of the market such as Recursion Pharmaceuticals, Pfizer, Novartis, Eli Lilly and Company, and Melior Discovery commanding a combined approximately 35% of global market share.
To strengthen their market positions, these companies are employing multi-faceted strategies including mergers and acquisitions, strategic alliances, and innovative pricing models. These efforts aim to enhance access to advanced therapies, improve affordability, and address unmet medical needs across diverse populations.
Simultaneously, emerging biotech firms and technology-driven startups are contributing to market evolution by introducing novel drug delivery systems such as subcutaneous formulations leveraging artificial intelligence for drug discovery. Their influence is particularly strong in regions like Asia-Pacific, Latin America, and the Middle East, where growing healthcare infrastructure and rising prevalence of chronic and rare genetic diseases are accelerating adoption.
As competition intensifies and therapeutic approaches diversify, companies are evolving their portfolios to meet global demand for effective, scalable, and personalized solutions. This ongoing innovation is fueling sustained growth and transforming the landscape of drug repurposing worldwide.
Drug Repurposing Market Companies
The company profile section includes both companies that have commercial drug available in the market as well as those that are onto clinical phase development. Prominent players operating in the market are as mentioned below:
- AbbVie
- Amgen
- Boehringer Ingelheim
- Cyclica
- Eli Lilly and Company
- GlaxoSmithKline
- Melior Discovery
- Novartis
- Pfizer
- Pharnext
- Recursion Pharmaceuticals
- Revolution Medicines
- Sanofi
- Schwarz Pharma
- Valence Discovery
- Recursion Pharmaceuticals
Recursion Pharmaceuticals leads the drug repurposing market with a share of ~12.5% in 2024. Recursion partnerships with Bayer and Roche validate its platform’s scalability. Its pipeline includes promising candidates like REC-617 (CDK7 inhibitor) and REC-4881 (MEK1/2 inhibitor), positioning it as a leader in precision repurposing for oncology and rare diseases.
Pfizer’s patient-centric marketing, robust pipeline, and commitment to affordability through cost leadership and differentiated therapies make it a dominant force in both developed and emerging markets.
The company’s global footprint, strong financials, and commitment to equitable healthcare access support its repurposing initiatives. Novartis also invests heavily in digital health and sustainability, reinforcing its competitive edge in scalable, patient-focused drug development across multiple therapeutic areas.
Drug Repurposing Market Industry News
- In April 2025, Qualthera Health Corporation, dedicated to building a nationwide network of quality, patient-centered compounding pharmacies, announced the official launch of Qualthera Pharmaceuticals Ltd. Co., a research-driven subsidiary dedicated to drug repurposing and innovative delivery systems. This expansion marks a significant step forward in accelerating pharmaceutical innovation, leveraging real-world patient insights to develop new therapies with streamlined development.
- In August 2024, Rejuvenate Biomed and SAS announced a partnership to develop a user-friendly drug repurposing discovery tool for researchers and scientists to uncover medically relevant insights about drugs, diseases, and biological networks, through fast and systematic explorations of hidden biological patterns. The discovery tool will be developed on SAS Viya, a powerful cloud-native artificial intelligence (AI) and data platform. The partnership will unite Rejuvenate Biomed’s clinically validated AI-driven drug discovery platform and end-to-end drug development proficiency with SAS’ decades of AI expertise.
- In September 2022, REMEDi4ALL, an ambitious EU-funded research initiative, launches to drive forward the repurposing of medicines in Europe. This promising approach to drug development consisting in the identification, testing, and validation of new therapeutic indications for existing medications, is a developing field but faces numerous barriers and systemic inefficiencies.
The drug repurposing market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2021 - 2034 for the following segments:
Market, By Drug Type
- Small molecules
- Biologics
Market, By Therapeutic Area
- Oncology
- Neurology and CNS disorders
- Infectious diseases
- Cardiovascular diseases
- Metabolic disorders
- Other therapeutic areas
Market, By Route of Administration
- Oral
- Intravenous
- Other routes of administration
Market, By End Use
- Pharmaceutical and biopharmaceutical companies
- Contract Research Organization (CROs)
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
What are the upcoming trends in the drug repurposing industry?
Key trends include the adoption of AI-driven platforms, increasing focus on rare and orphan diseases, and the dominance of oncology and CNS disorders as primary therapeutic areas.
Which region leads the drug repurposing market?
North America led the market with a 48.2% share in 2024, supported by strong regulatory frameworks and advanced healthcare infrastructure.
Which route of administration led the drug repurposing market in 2024?
The oral route of administration dominated the market in 2024, generating USD 18.4 billion in revenue.
What was the valuation of the oncology segment in 2024?
The oncology segment dominated the market with a revenue of USD 12.3 billion in 2024, driven by its prominence as a leading therapeutic area.
What was the revenue generated by the small molecules segment in 2024?
The small molecules segment accounted for 67.5% of the market in 2024. It is projected to exceed USD 33.8 billion by 2034, growing at a CAGR of 4.5% during the forecast period.
What is the projected size of the drug repurposing market in 2025?
The market is expected to reach USD 33.8 billion in 2025.
What is the projected value of the drug repurposing market by 2034?
The market is expected to reach USD 51.1 billion by 2034, fueled by advancements in AI-powered platforms, real-world evidence solutions, and growing focus on rare and orphan diseases.
What is the market size of the drug repurposing in 2024?
The market size was USD 32.6 billion in 2024, with a CAGR of 4.7% expected through 2034, driven by the increasing prevalence of chronic diseases, aging populations, and demand for cost-effective therapies.
Who are the key players in the drug repurposing market?
Key players include AbbVie, Amgen, Boehringer Ingelheim, Cyclica, Eli Lilly and Company, GlaxoSmithKline, Melior Discovery, Novartis, Pfizer, and Pharnext.
Drug Repurposing Market Scope
Related Reports


