Summary
Table of Content

Continuous Manufacturing Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Continuous Manufacturing Market Size
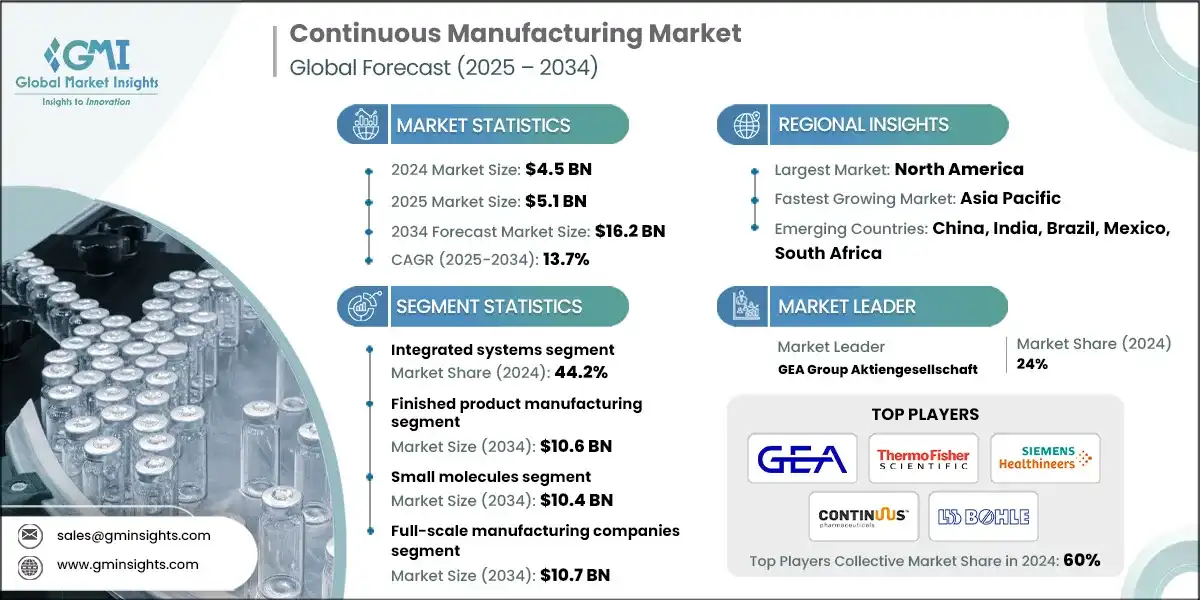
The global continuous manufacturing market was valued at USD 4.5 billion in 2024. The market is expected to grow from USD 5.1 billion in 2025 to USD 16.2 billion in 2034, at a CAGR of 13.7% during the forecast period, according to the latest report published by Global Market Insights Inc.
To get key market trends
The continuous manufacturing market is witnessing robust growth, driven by increasing demand for efficient, high-quality, and scalable pharmaceutical production. With regulatory bodies actively supporting the shift from batch to continuous processes, manufacturers are embracing integrated systems that offer superior control, reduced waste, and faster turnaround times. As industry moves toward more personalized and small-batch therapies, continuous manufacturing is emerging as a key enabler of flexible, patient-centric production. The growing emphasis on real-time quality monitoring and operational efficiency is further accelerating adoption across both small molecule and biologics segments.
This market segment is transforming pharmaceutical production by enabling faster, more efficient, and highly controlled manufacturing workflows. Leading companies such as GEA Group Aktiengesellschaft, Thermo Fisher Scientific, Siemens Healthineers, Continuus Pharmaceuticals, and L.B. Bohle Maschinen und Verfahren are at the forefront of this transformation. These players maintain their competitive edge through innovations in integrated systems, real-time monitoring technologies, and AI-powered software platforms. Their strategic focus on improving product quality, reducing operational costs, and supporting regulatory compliance is accelerating the adoption of continuous manufacturing across both small molecule and biologics production environments.
The continuous manufacturing market is witnessing sharp growth, expanding from USD 3.3 billion in 2021 to USD 4 billion in 2023. This growth is closely tied to the increasing push for modernization in pharmaceutical production. Regulatory bodies are actively supporting the adoption of continuous manufacturing due to its potential to enhance product quality, reduce waste, and improve supply chain resilience. The shift is further fueled by the rising demand for personalized therapies and small-batch production, which require flexible and efficient manufacturing systems. As pharmaceutical companies seek to streamline operations and meet evolving patient needs, continuous manufacturing is emerging as a transformative solution across both small molecule and biologics segments.
Moreover, the pursuit of operational efficiency and cost reduction is a major driver of growth in the continuous manufacturing market. Traditional batch manufacturing often involves lengthy production cycles, significant material waste, and complex quality control procedures. In contrast, continuous manufacturing streamlines production by integrating unit operations into a seamless flow, reducing downtime and improving resource utilization. This shift enables pharmaceutical companies to lower production costs, accelerate time-to-market, and respond more flexibly to demand fluctuations. As the industry faces increasing pressure to optimize margins and scale personalized therapies, continuous manufacturing is becoming a strategic imperative for both innovators and generic drug producers.
Continuous manufacturing systems are advanced pharmaceutical production technologies designed to streamline the end-to-end manufacturing of solid dosage forms such as tablets and capsules. These systems integrate multiple unit operations such as blending, granulation, drying, and compression into a seamless, uninterrupted process. By leveraging automation, real-time monitoring, and process analytical technologies (PAT), continuous manufacturing enhances precision, consistency, and efficiency in drug production. This approach significantly reduces manual intervention, production time, and waste, making it a transformative solution for modern pharmaceutical workflows.
Continuous Manufacturing Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 4.5 Billion |
| Market Size in 2025 | USD 5.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 13.7% |
| Market Size in 2034 | USD 16.2 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Regulatory support for continuous manufacturing | Regulatory agencies such as the FDA and EMA are actively promoting continuous manufacturing (CM) through guidance documents and expedited approval pathways, encouraging pharmaceutical companies to adopt CM for improved product quality and supply chain resilience. |
| Operational efficiency and cost reduction | CM enables streamlined production with fewer interruptions, reducing waste, energy consumption, and labor costs. This efficiency translates into significant long-term savings and faster time-to-market for pharmaceutical products. |
| Operational efficiency and cost reduction | Advanced analytics and process control technologies integrated into CM systems allow for real-time monitoring and quality assurance, minimizing batch failures and ensuring consistent product quality. |
| Rising demand for personalized and small-batch therapies | The shift toward precision medicine and niche therapies is driving demand for flexible manufacturing solutions. CM supports scalable production of small batches tailored to individual patient needs, making it ideal for personalized treatments. |
| Pitfalls & Challenges | Impact |
| High initial capital investment | The transition from batch to continuous manufacturing requires substantial upfront investment in equipment, infrastructure, and process redesign, which can be a barrier for small and mid-sized firms. |
| Limited skilled workforce | Implementing and maintaining CM systems demands specialized expertise in process engineering, automation, and data analytics. The shortage of trained professionals can hinder adoption and operational success. |
| Opportunities: | Impact |
| Expansion in biologics and large molecule manufacturing | As biologics gain prominence, CM technologies are being adapted to handle complex molecules, offering opportunities for innovation in upstream and downstream processing of biologics. |
| Growth in contract manufacturing services | CM is increasingly being adopted by contract development and manufacturing organizations (CDMOs) to offer flexible, high-quality production capabilities to pharmaceutical clients, expanding service portfolios and market reach. |
| Market Leaders (2024) | |
| Market Leaders |
24% market share |
| Top Players |
Collective market share in 2024 is 60% |
| Competitive Edge |
|
| Regional Insights | |
| Largest market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Continuous Manufacturing Market Trends
- The global pharmaceutical manufacturing landscape is undergoing a significant transformation, with continuous manufacturing (CM) emerging as a key enabler of efficiency, quality, and agility. This shift is driven by the growing demand for faster drug development, rising complexity of therapies, and the need for resilient supply chains. Regulatory bodies such as the FDA and EMA are actively supporting CM adoption through guidance frameworks and expedited approval pathways.
- In response, pharmaceutical companies are increasingly transitioning from traditional batch processes to continuous, end-to-end production models. CM systems offer substantial advantages, including reduced production time, real-time quality monitoring, and lower operational costs. These benefits are particularly valuable in the production of personalized medicines, small-batch therapies, and biologics, where flexibility and precision are critical.
- Technological innovation is a major catalyst in this market. Leading players are integrating process analytical technologies (PAT), AI-driven control systems, and modular equipment to enable seamless, data-rich manufacturing environments. For example, GEA’s ConsiGma platform exemplifies how automation and predictive analytics are reshaping pharmaceutical production.
- The demand for agile, scalable, and compliant manufacturing solutions is rising, especially among contract development and manufacturing organization (CDMOs) and biotech firms aiming to accelerate time-to-market. Continuous manufacturing enables on-demand production, minimizes human error, and enhances product consistency making it a strategic imperative for the future of pharmaceutical innovation.
Continuous Manufacturing Market Analysis
Learn more about the key segments shaping this market
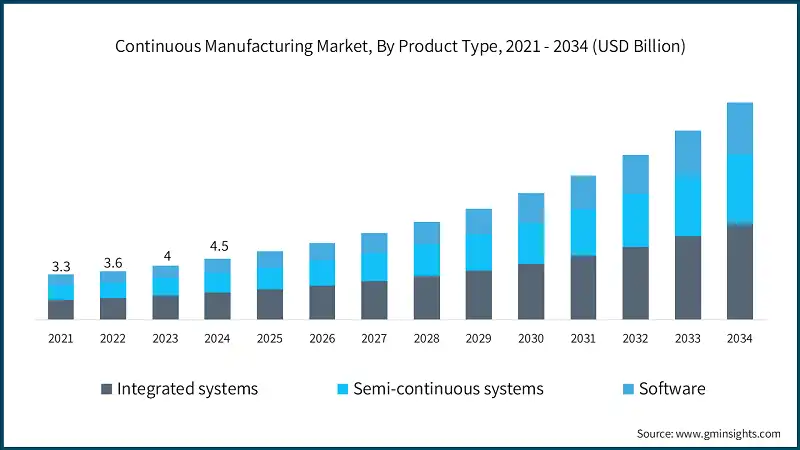
Based on product type, the market is divided into integrated systems, semi-continuous systems and software. The integrated systems segment accounted for 44.2% of the market share in 2024 primarily due to their ability to offer end-to-end automation, real-time quality control, and seamless scalability. The segment is expected to exceed USD 7.1 billion by 2034, growing with a CAGR of 13.7% through 2034. On the other hand, software segment is expected to grow with 14.2% CAGR through 2034. The growth of this segment is primarily driven by the increasing integration of advanced analytics, AI-based process control, and real-time monitoring tools into continuous manufacturing workflows.
- The integrated systems segment is the leading component of the global continuous manufacturing market. This dominance is attributed to their ability to deliver end-to-end, fully automated production workflows, combining material feeding, blending, granulation, drying, tableting, and coating into a single, seamless process. These systems are especially favored by large pharmaceutical manufacturers aiming to reduce production time, enhance product consistency, and ensure regulatory compliance.
- Integrated systems segment is valued for their high throughput, real-time quality control, and minimal manual intervention. They enable continuous, uninterrupted production, which significantly reduces downtime and batch variability. As the demand for agile and scalable manufacturing grows particularly for personalized medicines and small-batch biologics these systems are becoming central to next-generation pharmaceutical operations.
- Companies are enhancing system capabilities with AI-powered process control, digital twins, and advanced PAT (Process Analytical Technology) tools. For instance, Continuus Pharmaceuticals offers fully integrated continuous manufacturing platforms that combine drug substance and drug product operations, enabling rapid, flexible, and cost-effective production. Their Integrated Continuous Manufacturing (ICM) approach exemplifies how modular, closed-loop systems can revolutionize pharmaceutical supply chains.
- The segment’s expansion is also supported by increasing regulatory support and industry-wide digital transformation. As pharma companies seek to modernize legacy infrastructure and meet evolving quality standards, integrated systems offer a future-ready solution that aligns with both efficiency goals and compliance requirements.
Based on applications, continuous manufacturing market is classified into finished product manufacturing and API manufacturing. The finished product manufacturing segment dominated the market in 2024 and is expected to reach USD 10.6 billion by 2034.
- The finished product manufacturing segment is the leading application area in the global continuous manufacturing market, dominating in 2024. This growth is driven by the increasing adoption of continuous processes for producing oral solid dosage forms, injectables, and other final pharmaceutical products with greater speed, consistency, and cost-efficiency.
- Continuous manufacturing systems are transforming how finished pharmaceutical products are made by enabling real-time quality control, reduced production cycles, and minimal manual intervention. These systems streamline the entire production line from blending and granulation to tableting and coating ensuring uniformity and reducing the risk of batch failures.
- The demand for faster time-to-market, especially for personalized medicines and small-batch therapies, is pushing manufacturers to adopt integrated continuous platforms. These platforms support on-demand production, reduce inventory costs, and enhance supply chain agility key advantages in today’s dynamic pharmaceutical landscape.
- The segment is also benefiting from regulatory encouragement and global health priorities that emphasize quality, efficiency, and accessibility. As pharmaceutical companies modernize their manufacturing infrastructure, finished product continuous manufacturing is poised to become the new standard for high-quality, patient-centric drug production.
Based on therapeutic type, continuous manufacturing market is classified into small molecules and large molecules. The small molecules segment dominated the market in 2024 and is expected to reach USD 10.4 billion by 2034.
- The small molecules segment is the dominant therapeutic category in the global continuous manufacturing market, accounting for the largest revenue share in 2024. This leadership is driven by the widespread use of small molecule drugs in chronic disease management, infectious diseases, and oncology, as well as their compatibility with continuous manufacturing platforms.
- Small molecule drugs are well-suited for continuous production due to their relatively simple chemical structures, established formulation pathways, and high-volume demand. Continuous systems enable efficient synthesis, formulation, and packaging of these drugs, significantly reducing production time and improving batch-to-batch consistency.
- The segment benefits from technological advancements in process control, real-time analytics, and modular equipment design. These innovations allow manufacturers to optimize reaction conditions, monitor critical quality attributes, and scale production rapidly. Companies are pioneering integrated platforms that streamline the entire lifecycle of small molecule drug production from API synthesis to finished dosage forms.
- With the growing demand for cost-effective, high-quality therapies, especially in emerging markets, the small molecules segment is expected to remain the backbone of continuous manufacturing adoption. Its scalability, flexibility, and proven success across therapeutic areas make it a cornerstone of future pharmaceutical innovation.

Learn more about the key segments shaping this market
Based on end use, continuous manufacturing market is classified into R&D departments and full-scale manufacturing companies. The full-scale manufacturing companies segment dominated the market in 2024 and is expected to reach USD 10.7 billion by 2034. The full-scale manufacturing companies’ segment is further segmented into contract manufacturing organizations (CMOs) and pharmaceutical and biotechnological companies.
- The full-scale manufacturing companies’ segment is the dominant end use category in the global continuous manufacturing market, leading in 2024. This dominance is driven by the growing need for high-volume, cost-efficient, and quality-assured drug production across global pharmaceutical supply chains.
- Large pharmaceutical manufacturers are increasingly adopting continuous manufacturing systems to streamline operations, reduce production costs, and enhance product consistency. These systems enable uninterrupted workflows, real-time quality monitoring, and faster batch release, making them ideal for scaling up commercial drug production.
- The shift toward digital transformation and regulatory compliance is pushing full-scale manufacturers to invest in integrated platforms that combine automation, AI-driven process control, and advanced analytics. These technologies support predictive maintenance, reduce human error, and ensure adherence to stringent quality standards.
- The increasing demand for global drug accessibility, coupled with the need for resilient and scalable manufacturing models, is expected to further strengthen this segment. As pharmaceutical companies expand their production capabilities to meet evolving healthcare needs, full-scale continuous manufacturing will remain a cornerstone of future-ready operations.
Looking for region specific data?
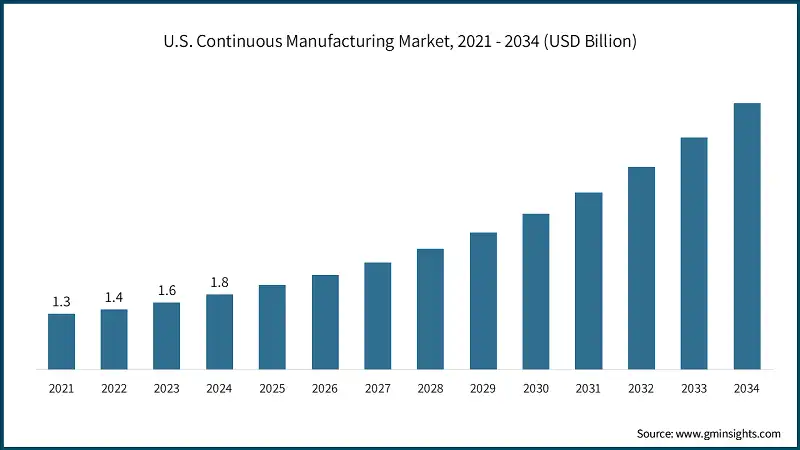
North America Continuous Manufacturing Market The U.S. continuous manufacturing market was valued at USD 1.3 billion and USD 1.4 billion in 2021 and 2022, respectively. The market size reached USD 1.8 billion in 2024, growing from USD 1.6 billion in 2023. The European market accounted for USD 1.2 billion in 2024 and is anticipated to show lucrative growth over the forecast period. Germany dominates the European continuous manufacturing market, showcasing strong growth potential. The Asia Pacific market is anticipated to grow at the highest CAGR during the analysis timeframe. China continuous manufacturing market is estimated to grow with a significant CAGR, in the Asia Pacific continuous manufacturing market. Brazil leads the Latin American continuous manufacturing market and is expected to exhibit remarkable growth during the analysis period. The Saudi Arabia market for continuous manufacturing is expected to experience substantial growth in the Middle East and Africa continuous manufacturing market in 2024. The continuous manufacturing industry is characterized by a mix of established global leaders and specialized technology providers, resulting in a dynamic and innovation-driven competitive landscape. The top five companies GEA Group Aktiengesellschaft, Thermo Fisher Scientific, Siemens Healthineers, Continuus Pharmaceuticals, and L.B. Bohle Maschinen und Verfahren collectively account for approximately 60% of the global market share. Their leadership is anchored in comprehensive product portfolios, global reach, and sustained investment in automation, AI integration, and modular system design. To strengthen their market positions, these companies are pursuing multi-pronged strategies, including strategic collaborations with pharmaceutical manufacturers and CDMOs, expansion into emerging markets, and continuous enhancement of their platforms. Their focus on end-to-end integrated systems, real-time process monitoring, and regulatory-compliant solutions is helping pharma companies achieve faster production cycles, improved quality control, and greater operational efficiency. Overall, the market is witnessing intensified competition and greater diversity, as both established and emerging players evolve their offerings to meet the global demand for efficient, high-quality, and digitally enabled pharmaceutical production. Prominent players operating in the continuous manufacturing industry are as mentioned below: Continuus Pharmaceuticals is a pioneering force in the continuous manufacturing market, known for its Integrated Continuous Manufacturing (ICM) platforms that combine drug substance and drug product operations. The company focuses on end-to-end solutions that enable rapid, flexible, and cost-effective production, particularly for small molecules and personalized therapies. Its commitment to innovation, regulatory alignment, and scalable design makes it a preferred partner for pharma companies seeking to modernize their manufacturing infrastructure. L.B. Bohle is a key contributor to the continuous manufacturing market, offering a wide range of modular systems for blending, granulation, drying, and tablet coating. The company is recognized for its precision engineering, process reliability, and ability to support both pilot-scale and full-scale production. Its continuous manufacturing solutions are widely adopted across Europe and Asia, helping pharmaceutical firms achieve consistent quality and streamlined operations. KORSCH plays a significant role in the continuous manufacturing space. The company provides engineering solutions that integrate seamlessly with upstream and downstream CM systems, enabling uninterrupted production and real-time quality control. KORSCH’s focus on flexibility, automation, and GMP compliance has positioned it as a trusted provider for pharmaceutical manufacturers transitioning to continuous workflows.Europe Continuous Manufacturing Market
Asia Pacific Continuous Manufacturing Market
Latin American Continuous Manufacturing Market
Middle East and Africa Continuous Manufacturing Market
Continuous Manufacturing Market Share
Continuous Manufacturing Market Companies
Continuous Manufacturing Industry News
The continuous manufacturing research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Billion from 2021 - 2034 for the following segments:
Market, By Product Type
- Integrated Systems
- Semi-continuous systems
- Continuous granulators
- Continuous coaters
- Continuous blenders
- Continuous dryers
- Continuous compressors
- Other semi-continuous systems
- Software
Market, By Application
- Finished product manufacturing
- Solid dosage
- Semi solid dosage
- Liquid dosage
- API manufacturing
Market, By Therapeutic Type
- Small molecule
- Large molecules
Market, By End Use
- R&D departments
- Research institutes
- Contract research organizations (CROs)
- Full-scale manufacturing companies
- Contract manufacturing organizations (CMOs)
- Pharmaceutical and biotechnological companies
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Who are the key players in the continuous manufacturing market?
Key players include Continuus Pharmaceuticals, Coperion GmbH, FREUND CORPORATION, GEA Group Aktiengesellschaft, Gebrüder Lödige Maschinenbau, Gericke, Glatt, KORSCH, L.B. Bohle Maschinen und Verfahren, Munson Machinery, Scott Equipment company, Siemens Healthineers, STEER World, Sturtevant Inc, Syntegon Technology, and Thermo Fisher Scientific.
What are the upcoming trends in the continuous manufacturing market?
Key trends include adoption of AI-driven control systems, integration of process analytical technologies (PAT), expansion in biologics manufacturing, and increasing use of modular equipment for real-time monitoring and efficiency.
Which region leads the continuous manufacturing market?
North America leads the market, with the U.S. market valued at USD 1.8 billion in 2024. Strong regulatory support from the FDA and advanced manufacturing infrastructure fuel the region's dominance.
What is the projected market value for finished product manufacturing segment by 2034?
Finished product manufacturing dominated the market in 2024 and is expected to reach USD 10.6 billion by 2034.
What is the growth outlook for the software segment from 2025 to 2034?
The software segment is projected to grow at 14.2% CAGR through 2034, driven by integration of advanced analytics, AI-based process control, and real-time monitoring tools.
What is the market size of continuous manufacturing in 2024?
The market size was USD 4.5 billion in 2024, with a CAGR of 13.7% expected through 2034 driven by increasing demand for efficient, high-quality, and scalable pharmaceutical production.
What is the projected value of the continuous manufacturing market by 2034?
The continuous manufacturing market is expected to reach USD 16.2 billion by 2034, propelled by regulatory support, operational efficiency, and rising demand for personalized therapies.
What is the current continuous manufacturing market size in 2025?
The market size is projected to reach USD 5.1 billion in 2025.
How much revenue did the integrated systems segment generate in 2024?
Integrated systems accounted for 44.2% market share in 2024, with the segment expected to exceed USD 7.1 billion by 2034.
Continuous Manufacturing Market Scope
Related Reports


