Home > Healthcare > Healthcare IT > Surveillance Solutions > Pharmacovigilance Outsourcing Market
Pharmacovigilance Outsourcing Market Size By Service (Pre-marketing Pharmacovigilance Services {Clinical Pharmacovigilance Services, Case-Processing Services, Safety Data Management Services, Medical Review}, Post-marketing Pharmacovigilance Services {Pharmacovigilance Knowledge Process Outsourcing Services, IT Solutions and Services}), By Service Providers (Contract Research Organizations, Business Processing Outsourcing), Regional Outlook, Application Potential, Competitive Market Share & Forecast, 2020 – 2026
- Report ID: GMI3030
- Published Date: Mar 2020
- Report Format: PDF
Pharmacovigilance Outsourcing Market Size
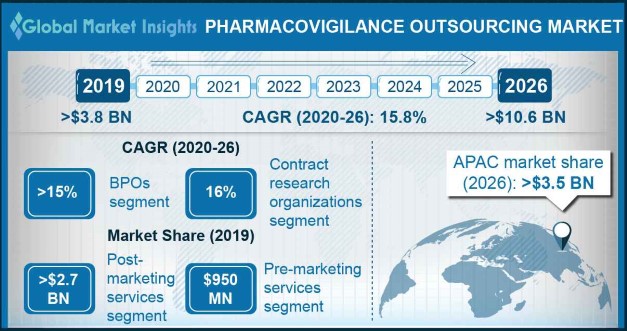
Pharmacovigilance Outsourcing Market size exceeded USD 3.8 billion in 2019 and is set to grow at around 15.8% CAGR between 2020 and 2026.
Pharmacovigilance (PV) can be defined as science and activities pertaining to the detection, understanding, assessment and prevention of drug-related problems. It plays a significant role in developing the healthcare system by assessing and monitoring the adverse drug interactions and their effects on human. The number of adverse drug reactions (ADRs) reported during the last few years has increased to a great extent and requires high level of expertise in pharmacovigilance to rapidly detect drug risks and defend the product against recalls.
| Report Attribute | Details |
|---|---|
| Base Year: | 2019 |
| Pharmacovigilance Outsourcing Market Size in 2019: | USD 3.8 Billion |
| Forecast Period: | 2020 to 2026 |
| Forecast Period 2020 to 2026 CAGR: | 15.8% |
| 2026 Value Projection: | USD 10.6 Billion |
| Historical Data for: | 2015 to 2019 |
| No. of Pages: | 225 |
| Tables, Charts & Figures: | 207 |
| Segments covered: | Service, Service Provider and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
Pharmacovigilance outsourcing refers to transfer of drug safety processes and functions to a third-party provider. These services are majorly outsourced by small and mid-size pharmaceutical and biotechnology companies with an aim to save costs and focus on company’s core activities. Some of the common PV activities that are generally outsourced include collecting ADR information, case processing activities, development of risk management plans & risk evaluation mitigation strategy as well as creating & submitting aggregate and expedited PV reports. Improved focus of companies on outsourcing certain drug safety processes to third party to lower costs and improve efficiency will escalate the market expansion.
Pharmacovigilance Outsourcing Market Analysis
Adverse drug reactions associated with pharmaceutical products used in prevention and treatment of various diseases will serve to be one of the major factors fostering the market growth. ADR monitoring is required for each drug throughout its life cycle, from drug development process including pre-marketing, early stages of drug design and clinical trials to post-marketing surveillance. Development in drug discovery process has led to availability of multiple new drugs in the market. Compliance of these drugs with the safety parameters laid by the regulatory authorities will upsurge the need for pharmacovigilance services.
Pharmaceutical and biopharmaceutical companies rely on pharmacovigilance activities that are performed in-house or are outsourced. Challenges in setting up in-house PV department such as high costs to maintain required levels of compliance, infrastructure and availability of qualified and trained in-house resources will lead to rise in outsourcing trends. Increasing number of pharmaceutical companies outsourcing their PV activities to service providers such as contract research organizations (CROs) and business process outsourcing (BPOs) will further spur the pharmacovigilance outsourcing market share.
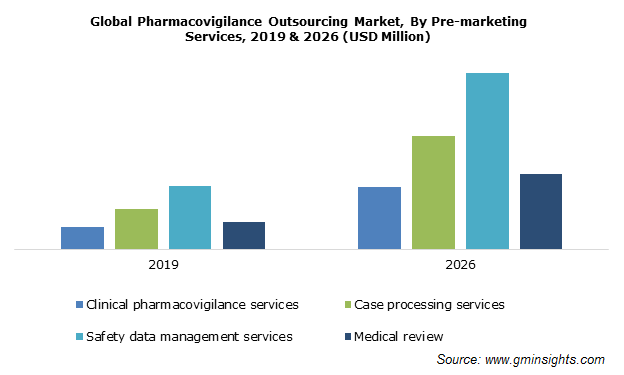
The pre-marketing services cover clinical pharmacovigilance services, case processing services, safety data management services and medical review. Post marketing services include knowledge process outsourcing services and IT solutions & services.
The pre-marketing services segment was valued around USD 950 million in 2019 and is estimated to grow significantly during the forecast period. Increasing focus of companies on product monitoring, clinical operations, regulatory affairs, statistical analysis, quality assurance, evaluation and drug approvals with necessary clearances and documentation will favor the pharmacovigilance outsourcing industry growth.
Pre-marketing surveillance involves data collection regarding adverse drug reactions from the pre-clinical screening to phases III clinical trials. Linking pre-marketing with human safety information is one of the emerging trends in pharmacovigilance outsourcing. Moreover, rising investments in R&D activities for developing computational approaches to predict potential ADRs using pre-clinical characteristics of the compounds or post-screening data will thus, prove beneficial for the market progress.
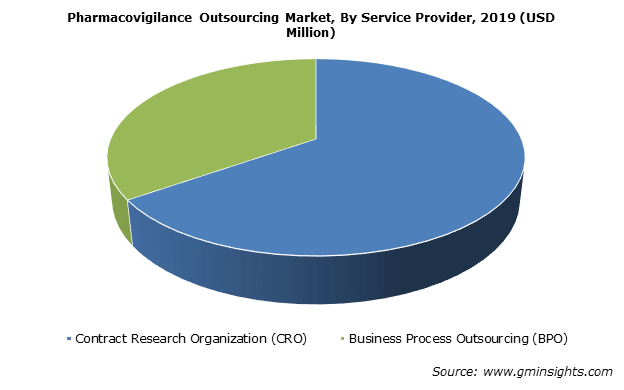
The contract research organizations segment of pharmacovigilance outsourcing market is anticipated to witness 16% CAGR over 2020 to 2026. Segment growth is attributed to dependence of pharma and biotech companies on CROs to carry out pharmacovigilance activities.
The small and medium-size pharmaceutical/biotechnology companies generally do not have a separate facility for performing PV activities to ensure drug safety. Outsourcing PV activities to CROs, reduce the cost of setting up an entire pharmacovigilance unit and enable time management for the small-size companies. Growing preference of drug safety teams towards outsourcing case management activities to CROs will thus, boost the market growth.
Asia Pacific industry is poised to exceed USD 3.5 billion revenue by 2026. Increasing volume of clinical trials being conducted in the Asian countries will serve to be a major impact rendering factor in the regional market growth. Developing countries such as China and India are the most favored destinations for PV outsourcing owing to availability of high skill set at lower costs. Moreover, availability of large pool of talented medical, paramedical and non-medical professionals involved in the PV process coupled with presence of refined PV systems in the region will further accelerate the market progression.
Pharmacovigilance Outsourcing Market Share
Some of the prominent players operating in the pharmacovigilance outsourcing market share include
- Accenture
- BioClinica
- Cognizant
- Covance
- ICON
- iGATE Corporation
- Genpact
- Infosys
- TCS
- Oracle
- PAREXEL
- Syneos Health
These industry players primarily focus on various inorganic strategies including partnerships, acquisitions and mergers to create a global footprint and sustain market competition.
Some of the recent industry developments:
- In April 2018, HCL Technologies announced acquisition of C3i Solutions, a leading service provider for life sciences and consumer packaged goods industries. The acquisition aimed at using C3i’s expertise in the clinical, pharmacovigilance domain to accelerate the Indian IT firm's growth in life sciences.
- In June 2017, Genpact launched AI based solution for transforming the drug safety processes that it outsources. Integration of the new technology in drug safety assessment has enabled the company to strengthen its existing portfolio and capitalize on market opportunities.
The pharmacovigilance outsourcing market research report includes an in-depth coverage of the industry with estimates & forecast in terms of revenue in USD from 2015 to 2026, for the following segments:
Click here to Buy Section of this Report
By Service
- Pre-marketing services
- Clinical pharmacovigilance services
- Case processing services
- Safety data management services
- Medical review
- Post-marketing services
- Knowledge process outsourcing services
- IT solutions and services
- Others
By Service Provider
- Contract Research Organization (CRO)
- Business Process Outsourcing (BPO)
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Asia Pacific
- China
- India
- Japan
- Australia
- Latin America
- Brazil
- Mexico
- Middle East & Africa
- South Africa
- Saudi Arabia
